Research Article - (2024) Volume 19, Issue 3
EFFECT OF HAPTIC ANCHOR SYSTEM ON REHABILITATION OF DYNAMIC BALANCE AND GAIT IN PATIENTS WITH CHRONIC STROKE
Shams K. A. Elbaz1*, Mohammed Sadek Badawy1, Amr Hassan2 and Ahmed S. Ali1,3*Correspondence: Shams K. A. Elbaz, Department of Physical Therapy for Neurology and Neurosurgery, Faculty of Physical Therapy, Cairo University, Egypt, Email:
2Professor of Neurology, Department of Neurology, Faculty of Medicine, Cairo University, Egypt
3Assistant Professor of Physical Therapy, Department of Physical Therapy and Health Rehabilitation, College of Applied Medical Sciences, Al-Qurayyat, J, KSA
Received: 23-May-2024 Published: 18-Jun-2024
Abstract
Background: Motor impairment and loss of balance and gait are the main factors affecting the independent function and activity participation of stroke patients. Haptic Anchor System is one of the modalities that can be used in physiotherapy and has a great effect on different impairments like improving postural control, trunk stability during balance tasks, and balance control during walking.
Objective: To determine the effect of the haptic anchor system on the rehabilitation of dynamic balance and gait in patients with chronic stroke.
Patients and Methods: Thirty patients with unilateral ischemic stroke of both sexes participated in this study, whose ages ranged between 45 to 60 years. They were randomly distributed into two equal groups: the control group (GA) and the study group (GB). The control group received a designed physical therapy program including a conventional balance and gait program, and the study group received the same designed physical therapy program given to the control group for balance and gait in addition to the haptic anchor system. The treatment was conducted for 12 sessions (2 sessions/week) 45 minutes for each session. The study was conducted a 6-week period in which all patients were assessed for dynamic balance and gait at baseline and after a 6-week intervention by using Biodex Balance System (BBS), Biodex gait trainer 2 TM treadmill, dynamic gait index (DGI) and timed up and go test (TUG).
Results: Comparison post-treatment results for both groups revealed statistically significant improvement of the step length, ambulation index, walking speed, LOS, TUG, APSI, and OASI in study group B compared to the control group A (P<0.05). Comparison between both groups revealed no significant difference regarding DGI (p = 0.33) and MLSI (p = 0.30) post-treatment.
Conclusion: Conventional balance and gait trainings alone may be not sufficient to improve balance and gait in chronic stroke patients .Adding the haptic anchor system to the rehabilitation program seems to be favorable approach in improving dynamic balance parameters (APSI, MLSI, OASI, and LOS) and gait parameters (step length, ambulation index, walking speed, DGI, and TUG) in the rehabilitation of patients with chronic stroke than conventional program alone.
Keywords
Dynamic balance, Gait, Haptic anchor system, Stroke. Biodex Balance System, Biodex gait trainer, Timed up and go test.
Introduction
According to Coupland et al. (2017), stroke is a neurological disorder that results from an immediate focused injury to the central nervous system (CNS) resulted from vascular issues including cerebral infarction, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage. It is a leading etiology of impairment and mortality globally.
Regarding to Rajsic et al. (2019), stroke is a major cause of morbidity and disability that entails high financial expenses for post-stroke care. According to Charvet et al. (2015), almost 50% of individuals who have had a persistent stroke experience motor impairment. Among these, one of the main functional deficiencies in stroke survivors has been found to be difficulty walking (Winstein et al., 2016). Stroke incidence varies throughout Middle Eastern nations, and the condition is steadily growing in importance (Habibi-koolaee et al., 2018).
The primary characteristics influencing stroke patients' ability to function independently and participate in activities are motor dysfunction, loss of balance, and gait (Zhang et al., 2021). Stroke survivors may experience significant consequences from walking difficulties, including diminished quality of life (QoL) and limited capacity to carry out daily tasks independently (Arienti et al., 2019).
Walking-related loss of balance is common following a stroke; according to Beyaert et al. (2015), 70% of stroke survivors report falling within a year of their stroke. Reduced walking speed is a hallmark of post-stroke gait and is caused by muscle weakness and lack of voluntary motions, which are common issues in the early stages of a stroke (Wonsetler and Bowden, 2017).
Moreover, a significant number of post-stroke patients have strong temporal and spatial inter-limb asymmetries, which range from 48% to 82% and 44% to 62%, respectively. These asymmetries are linked to poor standing balance control during locomotion (Boehm et al., 2016). Stroke patients' reduced preferred walking speed, stride length, and cadence are characteristics of their gait (Rinaldi and Monaco, 2013).
The anchor system was created by Mauerberg-Decastro in 2004 with the goal of enhancing physical stability. It functions as a haptic information mediator between the participant's body and the ground. By actively exploring the static or dynamic environment and interpreting spatiotemporal stimuli as they interrelate with various mechanoreceptors types, the haptic anchor system, also known as touch feedback, functions (Coelho et al., 2017).
Depending on the kinesthetic mechanoreceptors’ input in the arms and cutaneous mechanoreceptors in the fingers and hands, these haptic anchors offer data about the body's posture in relation to the supporting surface (Batistela et al., 2020). In both younger and older persons, the haptic information supplied by the anchors decrease trunk acceleration and enhances balance control during gait (Da Silva Costa et al., 2015).
The anchor system appears to improve the moving body's contact with its surroundings, allowing information regarding the body's direction with respect to the ground to be transmitted more easily. It has been demonstrated that the anchor system gives trunk stability during balancing exercises and enhances postural control in the elderly (Costa et al., 2018). There have been a few reports, nevertheless, regarding this system's advantages for those who have had chronic strokes.
Materials and Methods
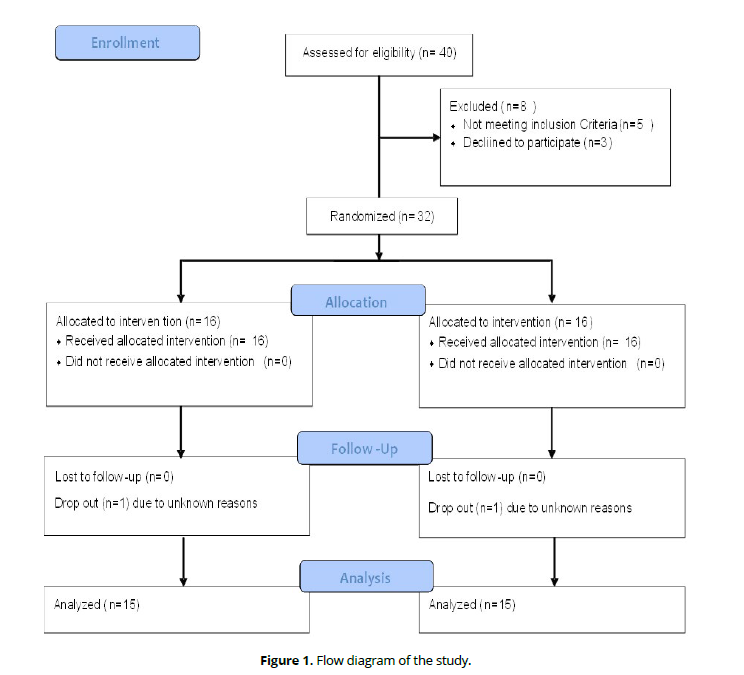
Out of 40 identified Egyptian stroke patients, only thirty patients completed the full requirements of the study. The flow diagram of the study is described in (Figure 1).
Trial design: A two-group pretest-posttest design, single-blinded randomized controlled trial. This study was conducted at the Outpatient Clinic for Neuromuscular Disorders and its Surgery at the Faculty of Physical Therapy, Cairo University, Egypt, which conducted from October 2022 to November 2023. The study was accepted by the local Ethics Committee of the Faculty of Physical Therapy number: P.T.REC/012/003781.
Participants: Thirty male and female patients with unilateral ischemic stroke were recruited, their age ranged from 45 and 60 years, the procedure and purposes of the study were explained to each participant before the beginning of the study. Then, each participant was asked to sign an informed consent. They were randomly assigned into two groups as the following: Control group A (n =15) received a designed physical therapy program including a conventional balance and gait program. Study group B (n =15) received the same designed physical therapy program given to the control group for balance and gait in addition to the haptic anchor system.
Inclusion criteria
Eligibility criteria included: Thirty adult post-stroke patients from both sexes were selected from the Outpatient Clinic of Physical Therapy for Neurology, Faculty of Physical Therapy, Cairo University, patients with first-onset unilateral ischemic stroke due to anterior cerebral artery occlusion, patients with good hand functions(able to grasp and release objects), medically stable and ambulant patients were recruited. The patients aged 45 to 60 years, their illness lasted from six months to one year, the spasticity degree in the lower limbs ranged from 1 to 1 + grade based on the modified Ashworth scale, grades of muscle tests for the lower limb muscles were 3/5 or more according to the manual muscle test. Patients without any other orthopedic deformity of the lower limb or neurological disorders that might affect the lower extremity motor function, and patients with good cognitive capabilities that allowed them to comprehend the study's requirements and who scored at least 24 out of 30 on the Mini-Mental State Examination were enrolled.
Exclusion criteria
The patients weren’t allowed to participate if they had: Recurrent strokes or hemiparesis due to other neurological causes rather than stroke, musculoskeletal problems (deformity or contracture) which might limit gait ability, exercise safety and performance are impacted by any cardiovascular conditions, including uncontrolled hypertension, severe coronary heart disease, and congestive heart failure, orthopedic disorders like severe degenerative osteoarthritis of hip, knee, and ankle joints, medically unstable or uncooperative patients, cognitive and psychological dysfunction, visual or auditory deficits and history of seizures.
Randomization
Patients were randomly distributed through simple randomization using closed envelopes into two equal treatment groups. Allocation to one of the two treatment options was revealed to the patients at the time of confirmation of enrolment. All participants were blinded to group allocation by ensuring that they were unaware of the exercises performed by the other group. To maintain the blinding, the intervention sessions were delivered separately to members of each treatment group.
Outcome Measures
The current study included two primary outcome measures: (1) Dynamic balance measures (anteroposterior stability , mediolateral stability, overall stability and limit of stability). (2) Gait measures (step length, walking speed, and ambulation index). The study compromised a 6-week period in which all patients were assessed for dynamic balance and gait at baseline and after a 6-week intervention by using Biodex Balance System (BBS), Biodex gait trainer 2 TM treadmill, dynamic gait index (DGI) and timed up and go test (TUG).
The primary outcomes recorded were as follows
Firstly, Biodex Balance System (BBS), it is a multi-axial instrument that measures and records a person's ability to keep their posture both in static and dynamic situations. A circular platform with anterior, posterior, medial, and lateral movement capabilities is part of the BBS setup. It can generate clinical data measurements that can be used for a variety of population types. Prior research has demonstrated its reliability as a tool for the objective evaluation of dynamic balance (Dawson et al., 2018). The following parameters were obtained: (1) overall stability (OAS); (2) anteroposterior stability (APS); (3) mediolateral stability (MLS); and (4) limit of stability (LOS). These parameters are obtained in dynamic conditions (Liu et al., 2021). For 20 seconds, participants in the dynamic standing condition had to stand with both legs extended on a moving platform. Each measure has been evaluated three times, with the averages being the results.
Secondly, Biodex gait trainer 2 TM treadmill: (model NO: 601-1-M90, EN60601 - USA) was designed particularly for evaluation of gait and gait training. The system consists of the following components: (1) An instrumented deck with kinematic gait metrics recorded and monitored. (2) The treadmill had a high-resolution color touchscreen LCD installed to control the device's settings and show the results. (3) Handrails. The instrument was used to evaluate the selected gait kinematic parameters before and after the treatment program including step length (M), walking speed (m/sec), and ambulation index (Gharib et al., 2011).Each patient was asked to catch firmly at the treadmill hand. The treadmill speed was adjusted to the fastest speed the patient was able to tolerate. The beginning test button was touched and the patient walked until time out.
Thirdly, Dynamic Gait Index (DGI): The purpose of the DGI is to evaluate the dynamic balance when walking. Eight items tested participants' ability to maintain their balance while walking normally and in multiple situations. The assignments were: 1) Walking on level surfaces. 2) Changing speeds (slow-fast). 3) Head turned in a horizontal direction. 4) Head turned in a vertical direction. 5) Walking and turning 180 degrees to stop. 6) Stepping over obstacles.7) Stepping around obstacles. 8) Stair climbing. The patient was graded by marking the lowest category that applies. Each component was scored 0 to 3 per item-specific scoring criteria. A maximum score of 24 was possible by grading each item from 0 to 3 as severely impaired, moderately impaired, mildly impaired, or normal. (Reoli et al., 2021). Time to administer: 10-15 minutes. A higher level of independent functional mobility is indicated by a higher total DGI score. (Alghadir et al., 2018).
Fourthly, Timed Up and Go Test (TUG): It also called the Test Stand and Walk, was an easy-to-use simple method of determining the risk of falls by timing a subject's movement from a seated position to a walking stance and back (Alves et al., 2014). The patient sat into a regular armchair, supported by the chair's arms and with his or her back against it. The patient turned around at the line, walked back to the chair, and took a seat. The line was three meters (9.8 ft) away. The patient's buttocks touching the seat signaled the end of the test. The patients were told to walk at a pace that was both safe and pleasant. The test was timed in seconds using a stopwatch.
Intervention
Haptic Anchor System: A simple, reasonably priced, and useful method for delivering haptic information was the haptic system. A tiny load (125 g) was fastened to one end of a flexible cable, which served as the anchor (Mauerberg et al., 2014). The load touches the ground and drags on the floor as the people walk, holding one anchor in each hand (Da Silva Costa et al., 2018). Through the decrease of information processing conflicts, the anchor system can assist the vestibular system in achieving a new modification of sensory mechanisms and/or enhance the somatosensory system, both of which improve balance (Coelho et al., 2017). It would be anticipated that a larger load (1,000 g) would offer more stability during the loss of equilibrium (Mauerberg et al., 2014). Given that patients with chronic stroke had a greater probability of falling and losing their balance, we recommended employing a 500 g load in our study.
The participants from both groups followed the training protocol twice a week for a period of 6 weeks. The sessions were supervised by the same researcher, ensuring consistency in the training process.
Control group A (G A): 15 Patients received a designed physiotherapy program for balance and gait program for 45 minutes for 12 sessions (2 sessions per week). The specifically designed training program was mostly composed of warm-up, self-stretching exercises for 10 minutes (trunk twists, trunk flexion, extension, lower limb stretching with active movements). Each exercise was carried out three times. Strengthening exercises for 10 minutes included lower extremity strengthening exercises (hip/knee flexion and extension, hip abduction and adduction, high-stepping, and squatting). Each exercise was performed for 10 repetitions. Balance exercises for 10 minutes included the following exercises: sit-to-stand (ten repetitions), standing on the affected side (30 sec each time) (5 repetitions), sideways walking toward the non-affected side (3 meters), Sitting on a Swiss ball with forward and side reaches (10 repetitions), standing on balance board exercise (10 sec, 5 repetitions), stride standing with eyes closed (30 sec, 5 repetitions) and gait training exercises for 15 minutes included the following exercises: A treadmill ambulation for seven minutes at a progressively faster pace that was self-selected. Endurance training includes obstacle crossing (30 seconds, 5 repetitions), figure-of-eight walking (3 meters), and stair climbing (3 meters) as progressions. (Kannan et al., 2020).
Study group B (G B):15 Patients received the same designed physiotherapy program as the control group as well as the haptic anchor system for 45 minutes for 12 sessions (2 sessions per week). The designed physiotherapy program was given for 20 minutes and the anchor system for 25 minutes. The haptic anchor system consists of a cable with a 500 g mass attached at one end. The 500 g mass was placed on one end of the cable, which each participant was instructed to hold in their hands. Due to the dynamic nature of the job, participants had to drag the anchors along with them while walking in order to maintain their contact with the ground and taut cables. According to Da Silva Costa et al. (2015), the participants felt variations in tension when they tugged the anchor cables, which gave them haptic information regarding their body's position in relation to the supporting surface. The therapeutic intervention followed Coelho and Abreu's protocol, which called for performing exercises to improve Balance: 1) Stride standing position for 30 seconds, 3 repetitions. 2) Standing with feet together for 30 seconds, 3 repetitions. 3) Single-leg stance for 30 seconds, 3 repetitions. 4) Anteroposterior trunk sway for 30 seconds, 3 repetitions. Gait Training: 1) Walking forward with anchors for 3 meters, 5 repetitions for each. 2) Tandem walking for 3 meters, 5 repetitions. 3) Walking over obstacles for 3 meters, 5 repetitions. 4) Walking backward with anchors for 3 meters, 5 repetitions. In order to keep the anchor stretched without removing the patient's weight from the floor, patients grasped it with their hands inside and their elbows bent between 60° and 90° (Coelho et al., 2019). • Patients were instructed to be in an erect posture, wore comfortable and loose-fitting clothes, and took deep breaths while performing exercises. The therapist stood beside patients during all exercises to take care of patients from falls and give correct instructions to perform exercises.
2.8Statistical analysis: The statistical SPSS Package program version 25 for Windows (SPSS, Inc., Chicago, IL) was utilized to conduct the statistical analysis. To compare the groups' age, weight, height, and BMI, an unpaired t-test was used. A chi-squared test was utilized to compare the distribution of the affected side and sex between the groups. To compare the effects of time (before Vs. after), treatment (between groups), and time and treatment interaction on step length, walking speed, ambulation index, DGI, TUG, balance indices, and LOS, a mixed MANOVA was performed. For subsequent multiple comparisons, post-hoc tests using the Bonferroni correction were utilized. A significance level of 0.05 was used for the data analysis.
Results
Subject characteristics
(Table 1) showed the subject characteristics of group A and B. There was no significant difference between groups regarding age, weight, height, BMI, sex and affected side distribution (p > 0.05).
| Group A | Group B | MD | t- value | p-value | |
|---|---|---|---|---|---|
| Mean ±SD | Mean ±SD | ||||
| Age (years) | 53.53 ± 3.42 | 53.13 ± 4.42 | 0.4 | 0.27 | 0.78 |
| Weight (kg) | 73.80 ± 7.45 | 75.60 ± 7.19 | -1.8 | -0.67 | 0.51 |
| Height (cm) | 165.13 ± 6.39 | 166.73 ± 7.62 | -1.6 | -0.62 | 0.53 |
| BMI (kg/m²) | 27.03 ± 1.89 | 27.27 ± 2.76 | -0.24 | -0.27 | 0.78 |
| Sex, N (%) | |||||
| Females | 6 (40%) | 7 (47%) | (χ2 = 1.36) | 0.71 | |
| Males | 9 (60%) | 8 (53%) | |||
| Affected side, N (%) | |||||
| Right side | 5 (33%) | 7 (48%) | (χ2 = 0.55) | 0.45 | |
| Left side | 10 (66%) | 8 (53%) |
Effect of treatment on step length, ambulation index, walking speed, DGI, TUG, balance indices and LOS
Mixed MANOVA revealed a significant interaction effect of treatment and time (F = 8.04, p = 0.001). There was a significant main effect of treatment (F = 2.61, p = 0.03). There was a significant main effect time (F = 56.13, p = 0.001).
Within group comparison: There was a significant increase in step length, ambulation index, walking speed and DGI and a significant decrease in TUG post-treatment compared with pretreatment in both groups (p > 0.05). The percent of change of step length, ambulation index, walking speed, DGI and TUG of group A was 13.63, 7.24, 18.60, 28.78 and 13.87% respectively; and that in group B was 28.57, 10.57, 54.76, 42.49 and 29.49% respectively (Table 2). There was a significant decrease in APSI, MLSI, OASI and a significant increase in LOS post-treatment compared with pretreatment in both groups (p > 0.01). The percent of change of APSI, MLSI, OASI and LOS of group A was 20.30, 13.93, 17.98 and 8.06% respectively; and that in group B was 39.90, 29.01, 38.53 and 27.68% respectively. (Table 3).
| Pre-treatment | Post-treatment | MD | % of change | p value | Sig | |
|---|---|---|---|---|---|---|
| Mean ±SD | Mean ±SD | |||||
| Step length (m) | ||||||
| Group A | 0.44 ± 0.11 | 0.50 ± 0.09 | -0.06 | 13.63 | 0.02 | S |
| Group B | 0.49 ± 0.13 | 0.63 ± 0.12 | -0.14 | 28.57 | 0.001 | S |
| MD | -0.05 | -0.13 | ||||
| p = 0.24 | p = 0.003 | |||||
| Ambulation index | ||||||
| Group A | 68.13 ± 5.01 | 73.06 ± 5.92 | -4.93 | 7.24 | 0.001 | S |
| Group B | 70.66 ± 5.91 | 78.13 ± 5.73 | -7.47 | 10.57 | 0.001 | S |
| MD | -2.53 | -5.07 | ||||
| p = 0.21 | p = 0.02 | |||||
| Walking speed (m/sec) | ||||||
| Group A | 0.43 ± 0.06 | 0.51 ± 0.08 | -0.08 | 18.6 | 0.001 | S |
| Group B | 0.42 ± 0.08 | 0.65 ± 0.09 | -0.23 | 54.76 | 0.001 | S |
| MD | 0.01 | -0.14 | ||||
| p = 0.94 | p = 0.001 | |||||
| DGI | ||||||
| Group A | 7.40 ± 1.54 | 9.53 ± 1.59 | -2.13 | 28.78 | 0.001 | S |
| Group B | 7.06 ± 1.43 | 10.06 ± 1.38 | -3 | 42.49 | 0.001 | S |
| MD | 0.33 | -0.53 | ||||
| p = 0.54 | p = 0.33 | |||||
| TUG (sec) | ||||||
| Group A | 28.33 ± 3.61 | 24.40 ± 4.11 | 3.93 | 13.87 | 0.001 | S |
| Group B | 27.60 ± 4.27 | 19.46 ± 3.87 | 8.14 | 29.49 | 0.001 | S |
| MD | 0.73 | 4.94 | ||||
| p = 0.61 | p = 0.002 |
| Pre-treatment | Post-treatment | |||||
|---|---|---|---|---|---|---|
| Mean ±SD | Mean ±SD | MD | % of change | p value | Sig | |
| APSI | ||||||
| Group A | 2.02 ± 0.50 | 1.61 ± 0.58 | 0.41 | 20.3 | 0.004 | S |
| Group B | 1.93 ± 0.76 | 1.16 ± 0.54 | 0.77 | 39.9 | 0.001 | S |
| MD | 0.09 | 0.45 | ||||
| p = 0.69 | p = 0.03 | |||||
| MLSI | ||||||
| Group A | 1.22 ± 0.42 | 1.05 ± 0.35 | 0.17 | 13.93 | 0.001 | S |
| Group B | 1.31 ± 0.32 | 0.93 ± 0.26 | 0.38 | 29.01 | 0.001 | S |
| MD | -0.09 | 0.12 | ||||
| p = 0.53 | p = 0.30 | |||||
| OASI | ||||||
| Group A | 2.28 ± 0.67 | 1.87 ± 0.33 | 0.41 | 17.98 | 0.01 | S |
| Group B | 2.31 ± 0.70 | 1.42 ± 0.22 | 0.89 | 38.53 | 0.001 | S |
| MD | -0.03 | 0.45 | ||||
| p = 0.89 | p = 0.001 | |||||
| LOS | ||||||
| Group A | 45.53 ± 8.70 | 49.20 ± 8.86 | -3.67 | 8.06 | 0.002 | S |
| Group B | 45.27 ± 10.46 | 57.80 ± 11.58 | -12.53 | 27.68 | 0.001 | S |
| MD | 0.26 | -8.6 | ||||
| p = 0.94 | p = 0.03 | |||||
Between group comparison: There was a significant increase in step length, ambulation index, walking speed and LOS of group B compared with that of group A post treatment (p < 0.05). There was a significant decrease in TUG, APSI and OASI of group B compared with that of group A post treatment (p < 0.05). There was no significant difference in DGI and MLSI between groups post treatment (p > 0.05) (Table 2-3).
Discussion
This study intended to explore and analyze the efficacy of the hepatic anchor system on the rehabilitation of dynamic balance and gait in patients with chronic stroke. A comparison was held between two equal groups; Group A (n =15) received a designed physiotherapy program for balance and gait program. Group B (n =15) received the same designed physiotherapy program as Group A as well as the hepatic anchor system. Before and after treatment, evaluation of all variables of balance (APSI, MLSI, OASI, and LOS) and gait variables (step length, ambulation index, walking speed, DGI, TUG) were conducted for each group. All the patients had similar baseline characteristics. There was no significant change in patient’s demographic data between both groups. And this indicates that the differences gained in this study were due to adding the haptic anchor system, and not due to the differences between groups concerning age, weight, height, BMI, sex, and affected side distribution. The inclusion Criteria of the patients were focused on having good hand functions, so the patients could hold the haptic anchor system with no difficulty. Also, Based on their Mini-Mental State Examination scores, patients should have adequate cognitive abilities to comprehend the study's requirements.
The results of the current study proved that there was a significant increase in the step length (P = 0.003), ambulation index (P = 0.02), walking speed (P = 0.001), LOS (P = 0.03) for the GB after-treatment compared with that of the GA. The results revealed also a significant decrease in TUG (P = 0.002), APSI (P = 0.03), and OASI (P = 0.001) in the GB after-treatment compared with that of the GA. There was no significant change in DGI (P = 0.33) and MLSI (P = 0.30) between both groups post-treatment.
The study's outcomes aligned with those of Brito et al.'s investigation on the impact of haptic anchoring on older individuals' balancing and walking activities (A systematic review). The study revealed that when older persons perform balancing and walking activities, haptic anchoring helps lessen postural sway. Postural sway is reduced as a result of using the anchors to examine the surroundings. The perception produced by muscle and skin mechanoreceptors enables one to learn about the characteristics of an object when it is held and moved (Brito et al.2023).
The study's results concurred with those of Freitas et al., who showed that the anchor system benefits were also noted in the study for older, healthier participants. Depending on how frequently (0%, 50%, and 100%) the subjects used the anchoring system while standing, they were split into three groups. When compared to a longer duration of anchor system use, postural control was enhanced during the shorter session duration at 50%. When the balancing test was completed without anchors 24 hours later, a brief two-day period of practice with anchors was sufficient to see a decrease in postural sway. The control group that did not use anchors did not show this reduction in postural sway. According to the authors, this outcome can be explained by the CNS using haptic information to adjust the sensory integration process for better postural control (Freitas et al., 2013).
According to Oates et al., it's critical to postpone mobility disability by using haptic feedback in an effort to improve walking in people with mobility difficulties. Step parameters during walking show an improvement in symmetry, and adding haptic input may help an individual recovering from a stroke become more active on the paretic side (gait velocity and step length increased) (Oates et al., 2017).
Acute application of a haptic anchor system decreased postural sway in the semi-tandem stance, as demonstrated by Brito et al. Furthermore, the anchor system increased the participants' perception of self-efficacy with reference to body sway postural control (Brito et al., 2022).
Unlike the current study, Moraes et al. compared the effects of the "light touch" and "anchor system" paradigms on postural sway and examined the impact of adding haptic information to posture control. The results indicated that although the anchors reduced postural sway, they did not improve postural control as much as light contact. The lack of improvement could be attributed to the anchoring paradigm's many degrees of freedom in its tasks (such as pulling upward, moving in the AP and ML directions, and rotating the hand or cable around a fixed contact on the ground in a various manners), which increases the exploratory system's redundancy (Moraes et al., 2018).
According to research by Mauerberg-de Castro et al., haptic anchoring is a type of "smart" perception that uses local interactions between the elements of an initially disordered system to self-organize behavior (postural-anchor-handling linked system). Both in non-visible ways (such as neural and non-neuronal plasticity) and observable ones (such as stability changes throughout experiments), the anchor system appears to support intrinsic adaptation. For the rehabilitation of neurodevelopmental problems and others, there is a need for valid intervention procedures (Mauerberg-deCastro et al., 2014). As a result, the anchor system can be a helpful tool in the rehabilitation of body balance programs. People with postural control deficits may find it challenging to do reaching tasks in the orthostatic position, like picking up something, or even when they get affected by an external force, if their stability limit is decreased. According to Freitas et al. (2013), an individual's capability to move without altering the support base decreases with decreasing stability limits, increasing the risk of fall-related injuries. The current study's findings demonstrated how patients with chronic strokes can have different walking control and dynamic balance when they walk with haptic anchor input. 4.1 Limitations
This study was conducted for a short term (six weeks) without patient follow-up to investigate the long term effects of the haptic anchor system on the rehabilitation of dynamic balance and gait in patients with chronic stroke.
Conclusions
The current evidence supports the positive impact of the haptic anchor system on the recovery of dynamic balance and gait in chronic stroke patients. The findings of the current study proved that adding the haptic anchor system to the rehabilitation program improved dynamic balance parameters (APSI, MLSI, OASI, and LOS) and gait parameters (step length, ambulation index, walking speed, DGI, and TUG) in the rehabilitation of patients with chronic stroke. Therefore, a haptic anchor system should be considered an integral part of the physiotherapy program for balance and gait training in stroke patients. This can help therapists to improve their rehabilitation program for chronic stroke patients to achieve better results and improve quality of life for the patients.
Funding
This study was not funded by any source
Declaration of Competing Interest
The authors declare no conflict of interest.
References
Coupland, A. P., Thapar, A., Qureshi, M. I., Jenkins, H., & Davies, A. H. (2017). The definition of stroke. Journal of the Royal Society of Medicine, 110(1), 9–12.
Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, Siebert U (2019). Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ 20:107–134.
Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, et al. (2015). Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci, 9(26), 325-338. https://doi.org/10.3389/fnsys.2015.00026
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research (2016). Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 47:e98–e169.
Habibi-Koolaee, M., Shahmoradi, L., Niakan Kalhori, S. R., Ghannadan, H., & Younesi, E. (2018). Prevalence of stroke risk factors and their distribution based on stroke subtypes in Gorgan: a retrospective hospital-based study—2015-2016. Neurology research international, 2018, 436-443. https://doi.org/10.1155/2018/2709654
Zhang, B., Li, D., Liu, Y., Wang, J., & Xiao, Q. (2021): Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta‐analysis. Journal of Advanced Nursing, 77(8), 3255-3273.
Arienti, C., Lazzarini, S. G., Pollock, A., & Negrini, S. (2019). Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PloS one, 14(7), e0219781.
Beyaert, C., Vasa, R., & Frykberg, G. E. (2015). Gait post-stroke: pathophysiology and rehabilitation strategies. Neurophysiologie Clinique/Clinical Neurophysiology ,45 (4-5), 335-355.
Wonsetler, E. C., & Bowden, M. G. (2017). A systematic review of mechanisms of gait speed change post-stroke. Part 2: exercise capacity, muscle activation, kinetics, and kinematics. Topics in stroke rehabilitation, 24(5), 394-403.
Boehm, W. L., & Gruben, K. G. (2016). Post-stroke walking behaviors consistent with altered ground reaction force direction control advise new approaches to research and therapy. Translational stroke research, 7(1), 3-11.
Rinaldi, L. A., & Monaco, V. (2013). Spatio-temporal parameters and intralimb coordination patterns describing hemiparetic locomotion at controlled speed. Journal of neuroengineering and rehabilitation, 10(1), 1-9.
Mauerberg-deCastro, E. (2004). Developing an “anchor” system to enhance postural control. Motor control, 8(3), 339-358.
Coelho, A. R., do Rego Andre, A. P., Perobelli, J. L., Sonobe, L. S., and de Abreu, D. C. (2017). Immediate effects of an anchor system on the stability limit of individuals with chronic dizziness of peripheral vestibular origin. Braz. J.Otorhinolaryngol. 83, 3–9. http://dx.doi.org/10.1016/j.bjorl.2015.12.008.
Batistela, R.A., Oates, A.R., Costa, A.A., Santos, L.O., & Moraes, R. (2020). Trunk balance control during beam walking improves with the haptic anchors without the interference of an auditory-cognitive task in older adults. Gait & posture, 81, 166-171.
Da Silva Costa, A. A., Manciopi, P. A. R., Mauerberg-deCastro, E., & Moraes, R. (2015). Haptic information provided by the “anchor system” reduces trunk sway acceleration in the frontal plane during tandem walking in older adults. Neuroscience letters, 609, 1-6.
Dawson N., Dzurino, D., Karleskint, M., & Tucker, J. (2018). Examining the reliability, correlation, and validity of commonly used assessment tools to measure balance. Health science reports, 1(12), e98.
Liu, Y.-T., Tsai, H.-T., Hsu, C.-Y., & Lin, Y.-N. (2021). Effects of orthopedic insoles on postural balance in patients with chronic stroke: A randomized crossover study. Gait & Posture, 87, 75–80. doi:10.1016/j.gaitpost.2021.04.014.
Gharib, N. M., El-Maksoud, G. M. A., & Rezk-Allah, S. S. (2011). Efficacy of gait trainer as an adjunct to traditional physical therapy on walking performance in hemiparetic cerebral palsied children: a randomized controlled trial. Clinical Rehabilitation, 25(10), 924-934.
Reoli, R., Therrien, A., Cherry-Allen, K., Keller, J., Millar, J., & Bastian, A. (2021). Is the dynamic gait index a useful outcome to measure balance and ambulation in patients with cerebellar ataxia? Gait & Posture, 89, 200-205.
Alghadir, A.H., Al-Eisa, E.S., Anwer, S. et al. (2018): Reliability,validity, and responsiveness of three scales for measuring balance in patients with chronic stroke. BMC Neurol 18, 141. https:// doi. Org/ 10.11 8 6/s12883-018-1146-9
Alves, Lucas Vieira, et al. (2014): Evaluation of the tendency to falls in elderly Sergipe. Revista CEFAC. v. 16, n. 5 ,pp. 1389-1396.
Mauerberg-de Castro, E., Moraes, R., Tavares, C. P., Figueiredo, G. A., Pacheco, S., & Costa, T. D. (2014). Haptic anchoring and human postural control. Psychology & Neuroscience, 7(3), 301-318.
Da Silva Costa, A. A., Dos Santos, L. O., Mauerberg-deCastro, E., & Moraes, R. (2018). Task difficulty has no effect on haptic anchoring during tandem walking in young and older adults. Neuroscience Letters, 666, 133-138.
Coelho, A. R., do Rego Andre, A. P., Perobelli, J. L., Sonobe, L. S., and de Abreu, D. C. (2017). Immediate effects of an anchor system on the stability limit of individuals with chronic dizziness of peripheral vestibular origin. Braz. J.Otorhinolaryngol. 83, 3–9. http://dx.doi.org/10.1016/j.bjorl.2015.12.008.
Coelho, A. R., Fontes, R. C., Moraes, R., Barros, C., & de Abreu, D. (2019). Effects of the Use of Anchor Systems in the Rehabilitation of Dynamic Balance and Gait in Individuals With Chronic Dizziness of Peripheral Vestibular Origin: A Single-Blinded, Randomized, Controlled Clinical Trial. Archives of physical medicine and rehabilitation, 101(2), 249–257. https://doi.org/10.1016/j.apmr. 07.0 12
Brito, T. S. S., Moraes, R., de Souza, L. A. P. S., & Luvizutto, G. J. (2023). Effectiveness of the haptic anchors during balancing and walking tasks in older adults: A systematic review. Journal of Bodywork and Movement Therapies, 35(2), 69-74.
Freitas, M. D. B. Z., Mauerberg-deCastro, E., & Moraes, R. (2013). Intermittent use of an “anchor system” improves postural control in healthy older adults. Gait & posture, 38(3), 433-437.
Oates, A. R., Hauck, L., Moraes, R., & Sibley, K. M. (2017). The effects of haptic input on biomechanical and neurophysiological parameters of walking: a scoping review. Gait & posture, 58, 232-239.
Brito, T. S., de Souza, L. A., & Luvizutto, G. J. (2022). Acute Effects of a Haptic Anchor System on Postural Sway of Individuals with Parkinson’s Disease: A Preliminary Study. Perceptual and Motor Skills, 129(6), 1775-1789.
Moraes, R., Bedo, B. L., Santos, L. O., Batistela, R. A., Santiago, P. R., & Mauerberg-deCastro, E. (2018). Additional haptic information provided by anchors reduces postural sway in young adults less than does light touch. Frontiers in Neuro science, 12, 326565.