Research - (2024) Volume 19, Issue 4
EFFECT OF MECHANICAL VESTIBULAR STIMULATION ON HAND FUNCTION IN CHILDREN WITH HEMIPARETIC CEREBRAL PALSY
Ahmed Mohamed Abdel Haleem1*, Abdel Aziz Ali Sherif2, Ibrahim Mohamed Hamoda3 and Sara Y. Elsebahy4*Correspondence: Ahmed Mohamed Abdel Haleem, Physiotherapist at Armed Forces Medical Complex in Alexandria, Egypt, Email:
2Dean of the Faculty of Physical Therapy Rashid University, Egypt
3Assistant Professor of Physical Therapy for Neurology and Neurosurgery Faculty of Physical Therapy Kafr El Sheikh University, Egypt
4Lecturer of Physical Therapy for Pediatrics Department of Physical Therapy for Pediatrics Faculty of Physical Therapy Kafr El Sheikh University, Egypt
Received: 10-Aug-2024 Published: 21-Aug-2024
Abstract
Objective: To investigate the effects of mechanical vestibular stimulation on fine motor skills and hand grip strength in children with hemiparetic cerebral palsy.
Methods: Sixty hemiparetic cerebral palsied children of both sexes, ranging in age from 4 to 6 years participated in this study. They are randomly assigned into two groups: control group (A) and study group (B). Children in group A received the designed physical therapy program. Children in group B received the same physical therapy program given to group A for 45 min then mechanical vestibular exercise for 15 mins. Fine motor skills, including visual motor integration, grasp and fine motor quotient evaluated using Peabody Developmental Motor Scale (PDMS-2) while hand grip strength measured using Jamar Handheld Dynamometer before and after three months of treatment.
Results: This study showed a statistically significant improvement in the two groups when comparing their pre and post treatment mean values of all measured variables. When comparing the post-treatment results, there were significant differences between groups A and B in favor of group B regarding all measured variables.
Conclusion: Mechanical vestibular stimulation is better added to the rehabilitation program to improve fine motor skills and hand grip strength in children with hemiparetic cerebral palsy
Keywords
Mechanical Vestibular Stimulation. Cerebral palsy. Fine Motor Skills. Hand Grip strength. Hemiparesis
Introduction
Cerebral palsy (CP) is the most common neurological condition that impairs muscle tone, posture, and movement. The underlying cause is injury to the growing brain during the prenatal to neonatal periods. Even if the underlying neuro pathologic lesion does not worsen, children with CP might acquire a variety of secondary conditions that can impair their functional abilities 1. CP is the most prevalent cause of physical impairment in early life, with two to three cases per 1000 live births 2.
Hemiparesis is a unilateral motor loss caused by a brain injury that results in more disability on one side of the body than the other. Patients with hemiparetic CP may exhibit more severe deficits in their upper limbs than in their lower limbs. Such upper limb malformaciones may severely impede a child's capacity to participate in daily activities and live a normal life 3.
Children with brain injuries that cause unilateral cerebral palsy (uCP) frequently have upper limb (UL) abnormalities such as sensory impairments, stiffness, synkinetic movements, coordination concerns, and motor planning difficulties. These deficits make it more difficult to grasp and manipulate items. Bimanual tasks (such as buttoning garments, tying laces, or opening bottles) are more difficult for children with uCP than for typical children because they require bilateral hand coordination and interaction 4.
The development of hand function is dependent on the development of the visual, perceptual, perceptual motor, and cognitive systems, as well as the motor control of the arms, hands, and shoulders. The primary motor components of hand function are the type of grasp, the reach pattern, the reach-and-grasp pattern, and the release pattern. These qualities can develop independently of gross motor activities. When a kid is in well-supported laying, sitting, or standing positions, he or she can focus on fine motor development, which is necessary for the development of upper limb function. Children with CP who were hemiplegic demonstrated severe impairment in upper extremity motor abilities 5.
The vestibular system plays an important role in the development of gross motor skills. It works with the visual and somatosensory systems to control movement and balance. The vestibular system, which also influences the motor system, has a considerable impact on children's cognitive function. This system enables children to understand their surroundings 6.
Fine motor skills may also be associated with vestibular function. To attain optimal hand-eye coordination, many fine motor activities require a functional vestíbulo-ocular reflex (VOR) that stabilized vision. It is widely acknowledged that the vestibular system helps to maintain postural stability and is involved in a variety of motor functions 7.
Vestibular stimulation can improve respiratory system vital signs and heart rate, balance, body orientations, sensory processing, learning, and neuromotor development, such as passive muscle tone, active mortality, posture, oral motor function, and neuromuscular maturity 8.
Thus, the study's purpose was to determine the effects of both the physical treatment program and mechanical vestibular stimulation on the hand function of children with hemiparetic cerebral palsy.
Subject and Methods
The study's objectives and protocol were designed in compliance with the recommendations adopted by the Declaration of Helsinki. Each parent gave written conscent for their child to participate in the examination and treatment plan. The Ethics Committee Board of the Faculty of Physical Therapy at Kafr El Sheikh University accepted the research project (P.T/PED/2/2023/30). Between December 2023 and March 2024, a study was done in the outpatient clinic of Kafr El Sheikh University's Faculty of Physical Therapy. The clinical trial registration number is NCT05782413.
Subjects
Sixty-five (65) hemiparetic CP children (defined by Hagberg classification as having prenatal and/or perinatal brain injury) were selected for the study; five were excluded because they did not meet the inclusion criteria. This study included sixty children aged four to six years with hemiparesis of both sexes (39 boys, 21 girls) with hand spasticity ranging from one to one plus on the Modified Ashworth Scale. They were chosen from the outpatient clinic at the Faculty of Physical Therapy at Kafr El Sheikh University. This study did not include children with moderate to severe spasticity, fixed upper-limb abnormalities, or mental disabilities. Children who met the preceding inclusion criteria were randomly allocated to one of two groups: control (A) or study (B). After 45 minutes of the same scheduled physical therapy program as children in control group A (18 boys, 12 girls), children in the study group B (21 boys, 9 girls) participated in an extra 15 minutes of mechanical vestibular exercise. Each group had three therapy sessions per week for a period of twelve weeks.
Randomization
The randomization was implemented using a computer-generated randomized table created with the SPSS application (version 27 for Windows). Each participant received an identity number. These numbers were divided into two equal-sized groups (n = 30). Index cards were Sequentially numbered and stored in opaque envelopes. A blinded researcher opened the sealed package and assigned the patients to respective groups (Figure 1).
Sample size estimation
Sample size
A two-tailed statistical analysis with a big effect size of 0.8 and α = 0.05 yielded an actual power of 80.75 (1-β). The impact size is calculated from a pilot study of eight people (4 in each group). The Computation yielded a sample size of 26 for each category. To exceed the expected dropout rate, each group's number will be increased by 15.38%, up to 30 people. The sample size was calculated using the application G*power (3.1.9.2).
Instrumentation
- Peabody developmental motor scale (PDMS-2)
This test evaluates the fine and gross motor skills of children aged 0 to 71 months. The PDMS-2 is currently used to monitor a child's growth, set specific goals and objectives for therapy or intervention, evaluate a child's motor competence, and compare it to standardized norms, and identify relative disparities in fine and gross motor development. The PDMS-2 criteria were constructed using data from 2003 children in 46 states and one province of Canada. The PDMS-2 outperformed the earlier version in terms of standard psychometric characteristics and representativeness 9.
- Jamar hand-held dynamometer
The American Society of Hand Therapists and other organizations recommend utilizing the Jamar handheld dynamometer to measure grip strength. It is a legal and trustworthy dynamometer as long as it is calibrated correctly and used according to proper positioning and instructions. Test-retest and inter-rater reliability have been reported to vary from good to exceptional. The availability of normative data for both adults and children makes the Jamar dynamometer more useful in clinical settings. Because of these properties, the Jamar dynamometer is now considered the "gold standard" for assessing grip strength 10.
Both groups were treated with occupational therapy instruments of varying sizes and shapes, such as balls, cubes, cards, dough, marbles, scissors, puzzles, keys, zipper, beads, toothbrush and paste, buttons, jar, lock, and key.
Procedures
- Assessment
1- Assessment of Fine Motor Quotient in PDMS-2
It includes 98 fine motor items divided into two subtests: the 26-item grasping subtest and the 72-item visual-motor integration subtests. These subtests evaluate how well the tiny muscles involved in gripping and visual-motor integration are employed. Its remarkable validity and durability make it a powerful weapon for discrimination.
Test Evaluation: The Peabody Developmental Motor Scale 2 is used as follows. When the kid completed the testing item and met the mastery criterion, they received a score of two. If the testing item was executed in a way that nearly met but did not fully fulfill the item mastery criteria, the child received a score of one. If the child fails to finish the task, they will receive a zero.
When presenting the assessment, utilize the following entry/start point, basal level, and ceiling level for each subtest:
The item administration procedure begins at the time of admission, which is defined by the age of the child. To move on, the youngster must score two on the first three consecutive objects. We stopped testing if the child scored (0) or (1) on any of the first three things completed beginning with the entry point and testing backward until the child scored 2 on three consecutive items (base level). Once the baseline was established, the examiner proceded to present increasingly harder tasks until a ceiling was reached. When a child receives a zero, his or her score on three consecutive items is referred to as the ceiling or ceiling level. A child could try each item up to three times before it was scored. Each item had three possible scores: zero, one, or two. The child's total raw score for each subtest was computed by summing the sum of the subtest item scores.
Interpreting the results: The scale contains five different score categories: raw, quotient for composites, percentile, age-equivalent, standard, and raw. The handbook's norm tables are used to calculate the standard scores, age-equivalent scores, and raw scores for each subtest.
Grip dynamometer (Jamar handheld dynamometer)
The data collection approach was changed for children aged 4 to 6 in accordance with the study's recommendation, and it included the use of Jamar handheld dynamometers. Each participant sat at a table or chair appropriate for their height, rotated and adducted their shoulders, bent their elbows to a 90°, and kept their forearm in a neutral position. The wrist was stretched between 0 and 30°, with an ulnar deviation of 0 to 15°. Throughout the procedure, the patient kept his untested hand on his lap. The dynamometer was placed on the table, and the subject pressed down while the research assistant held the top in place. Three grip measurements were collected, with a 30-second break in between. As a strong metric, the highest of the three metrics was selected.
- Treatment
Each group had three one-hour therapy sessions per week for three months in a succession. To enhance upper limb function, the control group's children engaged in a tailored exercise program for the affected upper extremity, based on NDT Bobath neurodevelopmental treatment and occupational therapy principles. The program included exercises such as reflex inhibiting exercises, hand weight bearing, using occupational therapy equipment to shape the damaged hand, and practicing repetitive activities such as rolling dice, rotating cards, and putting dough and marbles together.
Patients in the study group were allocated to the control group, which did 45 minutes of design exercise and 15 minutes of mechanical vestibular exercises.
Mechanical vestibular stimulation system
The equipment is designed to rotate in three dimensions, using a mechanical rotation mechanism. The device is rotatably attached to a frame. The vestibular stimulation devices described below are designed to rotate independently in two or three axes. The device is also programmed to rotate continuously along each axis of rotation in all 360 degrees of freedom at individually adjustable accelerations for vestibular stimulation.
Procedures of mechanical vestibular stimulation
The youngster was seated on the disc swing, his hands gripping the ropes at the sides, and the therapist stood behind him, pushing the platform back and forth, side to side, and spinning. Throughout, the toddler tried to keep his equilibrium. During the mechanical vestibular session, the patient pushed for five minutes in each direction, totaling fifteen minutes.
Statistical analysis
All statistical analyses were conducted using the statistical program for social studies (SPSS) version 27 for Windows. The Shapiro-Wilk test was used to assess the normality of data. Descriptive statistics and an unpaired t test were used to compare pre- and post-treatment hand grip strength measurements between the two groups, as well as pre-treatment fine motor quotient assessments. A paired t test was used to compare before and post treatment measurements of hand grip strength for each group. The Mann-Whitney U test was used to compare the post-treatment fine motor quotients between the two groups. The Wilcoxon Signed-Ranks test was used to compare pre- and post-treatment assessments of fine motor quotient for each group. All statistical tests had a level of significance of p < 0.05.
Results
Table 1 displays the clinical features and basic demographic data of the sixty people with hemiparetic CP. At the baseline examination, there were no statistically significant differences in age or sex between the control and study groups. Children in the study group were 4.95 ± 0.51 years old, compared to 5.41 ± 0.44 years in the control group. The study group had 30% girls and 70% boys, while the control group had 40% and 60% females, respectively (Table 1).
| Items | control group | Study group | Significance |
|---|---|---|---|
| mean ± SD | mean ± SD | ||
| Age (years) | 5.14 ± 0.47 | 4.95 ± 0.51 | NS |
| Girls/boys | 12/18 40%/60% | 9/21 30%/70% |
NS |
Comparison between groups before and after treatment
Hand grip strength
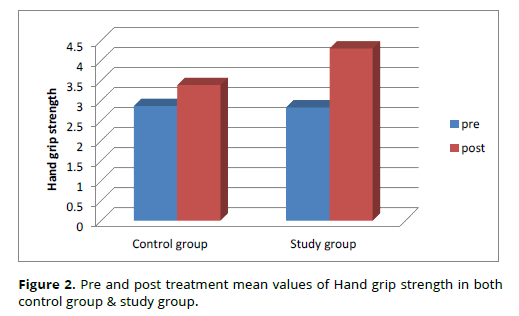
The pretreatment averages of hand grip strength in the study and control groups were 2.83 ± 0.99 and 2.87 ± 1, respectively, as shown in table (2) and figure (2), with a difference of 0.04. This meant that the differences between the two groups were statistically insignificant.
Before therapy, the study group's mean hand grip strength values were 2.87 ± 1 and 2.83 ± 0.99, respectively. After treatment, the mean values were 3.39 ± 0.92 and 4.3 ± 1.02. Following therapy, Table (2) and Figure (2) show that both groups improved significantly; the study group improved by 52 percent, while the control group improved by 18 percent.
The average post-treatment hand grip strength in the study and control groups was 4.3 ± 1.02 and 3.39 ± 0.92, respectively, as shown in table (2) and figure (2). A mean difference of 0.91 revealed a significant difference between the two groups (Table 2, Figure 2).
| Variables | pretreatment | post treatment | MD | % of change | test-value | p-value |
|---|---|---|---|---|---|---|
| Hand grip strength | ||||||
| Control group | 2.87 ± 1 | 3.39 ± 0.92 | 0.52 | 18% | t= 9.912 | 8.1 E -11 |
| (n = 30) | ||||||
| Study group | 2.83 ± 0.99 | 4.3 ± 1.02 | 1.47 | 52% | t=17.252 | 1.4 E -16 |
| (n = 30) | ||||||
| Mean difference | 0.04 | 0.91 | ||||
| test-value | t= 0.162 | t= 3.638 | ||||
| p-value | 0.872 | 0.0006 |
Fine motor quotient (FMQ)
The pre-treatment FMQ averages for the study and control groups were 63.6 ± 5.27 and 64.1 ± 5.49, respectively, as shown in table (3) and figure (3). A mean difference of 0.5 means there was no significant difference between the two groups.
Prior to therapy, the FMQ values for the study and control groups were 64.1 ± 5.49 and 63.6 ± 5.27, respectively. After therapy, the study and control groups had FMQ averages of 86.03 ± 5.44 and 74.8 ± 5.32, respectively. Table (3) and Figure (3) show that the study group improved significantly following treatment, with a percentage of thirty-five improvements compared to the control group's 17.
The study and control groups had post-treatment FMQ averages of 86.03 ± 5.44 and 74.8 ± 5.32, respectively, with a mean difference of 11.23, indicating a substantial difference between the two groups (Table 3, Figure 3).
Variables |
pretreatment | post treatment | MD | % of change | test-value | p-value |
|---|---|---|---|---|---|---|
| FMQ | ||||||
| Control group | 64.1 ± 5.49 | 74.8 ± 5.32 | 10.7 | 17% | z= - 4.782 | 1.7 E -6 |
| (n = 30) | ||||||
| Study group | 63.6 ± 5.27 | 86.03 ± 5.44 | 22.43 | 35% | z= - 4.782 | 1.7 E -6 |
| (n = 30) | ||||||
| Mean difference | 0.5 | 11.23 | ||||
| test-value | t= 0.36 | z= - 6.025 | ||||
| p-value | 0.72 | 1.69 E -9 |
Discussion
Children with hemiparesis may face delays in the development of fine motor abilities as well as other motor functions due to stiffness and muscle weakness. As a result, the children would have difficulties completing hand functions; thus, the current study was designed to explore the effects of mechanical vestibular stimulation on the children's hand function.
The diagnosis of hemiparetic cerebral palsy is consistent with Goyal et al.'s findings. The upper extremity, notably the hand, is more afflicted than the lower extremity in around half of children with hemiplegic cerebral palsy 11. According to Said et al., the most common motor deficit in children with hemiplegia is reduced upper extremity function, which has a substantial impact on everyday activities. In children with hemiplegia, the affected upper extremity's functional abilities are limited by aberrant tone, impaired motor selectivity, weakness, and stiffness 12.
Pre-treatment mean hand function values from the research and control groups indicate that a considerable number of the children in those groups experienced "hand functions problems such as eating, dressing, and bilateral hand use activities." According to Niazi et al., 13 hemiplegic cerebral palsy affects the upper limbs' capacity to contract, feel, and have powerful muscles. The muscles' ability to grab, reach, release, and move objects is frequently hampered as a result. It also causes issues with self-care, exercise, and training, lowering one's quality of life, self-esteem, confidence, and self-concept.
The way spasticity causes changes in muscle length may explain the current study's findings, which reveal a reduction in hand grip strength. This is supported by the findings of Abd El Wahab and Hamed 14, who found that muscle length influences muscle tension. As a result, if a person has spasticity and their muscle length falls below resting levels, their muscles' ability to generate force is reduced, affecting their grasping ability.
The significant improvement in post-treatment evaluated variables for both groups may be attributable to the impact of a typical physical and occupational therapy program based on non-directive therapy (NDT). The purpose of this program was to encourage good postural control patterns and broaden the spectrum of normal movement patterns, particularly in the upper limbs. This is congruent with findings by Te Velde et al. 15. They discovered that children who received NDT or a combination of NDT and other interventions fared better than those who received other services.
At the end of the treatment period, the research groups' hand grip strength improved significantly, which could be attributed to vestibular stimulation's ability to promote muscular growth and maintain normal muscle tone. This is consistent with the findings of Sailesh et al., 16 who discovered that vestibular stimulation inhibits flexor muscles and preferentially stimulates extensor or antigravity muscles. The vestibulo-spinal, vestibulo-ocular, and vestibulecolic pathways all play important roles in maintaining muscle tone.
Gains in fine motor abilities and the ability to hold an object in one hand before moving to controlled finger motions can be linked to the research groups significantly improved post-treatment grasp and VMI performance. It could also be related to vestibular input, which allows children to successfully interact with their surroundings through fine motor and visual motor skills (such as tracking a moving item). This supports the findings of Kuatsjah et al., 17 who found that the vestibular system, which is strongly multimodal and highly convergent with other sensory and motor signals, supports a variety of functions such as automatic reflexes, motor planning, spatial navigation, learning, memory, bodily self-consciousness, affective processes, and self-motion perception. The vestibular branch of the eight cranial nerves connects the vestibular end organs to the brainstem and cerebellum, where it projects to the vestibular nuclei. These vestibular nuclei integrate information from both the ocular and somatic sensory systems. Previous research has also employed the sensorimotor transformation paradigm to study the link between vestibular information and motor output. For example, vestibular impulses from the inner ear activate the vestibulo-ocular reflex, which shifts the eyes to stable pictures on the retina and compensates for even minor head movements during daily tasks. This reaction is required for gaze control to work effectively, which is dependent on interactions between visual, vestibular, and proprioceptive signals across the central vestibular pathways.
The current study's findings revealed a considerable improvement in the study groups' measured variables following the recommended treatment duration. The vestibular system is one of the sensory systems responsible for posture, equilibrium, orientations, and balance. During head movements, the vestibular system is responsible for maintaining posture and visual focus 18. Postural control is aligning the body in space to provide a stable foundation for function and task performance. The execution of symmetrical, controlled, and regulated upper extremity movement requires appropriate postural control 19.
Implications on practice
Hand function is a serious concern for children with hemiparetic cerebral palsy. As a result, the interventions employed for them should point in the right direction for enhancing this necessary physical function so that they can participate in active living activities.
Conclusion
Mechanical vestibular stimulation is an effective addition to hemiparetic cerebral palsy treatment regimens for improving hand function in children.
Recommendations
This study demonstrated how effectively mechanical vestibular stimulation can enhance hand function in cerebral palsy patients. As a result, these children should get mechanical vestibular stimulation as part of their rehabilitation program. The findings of this study may assist patients with HCP who have hand impairments in improving their hand function.
Ethics approval
This study was approved by the Ethical Committee of the Faculty of Physical Therapy, Kafr El Sheikh University, Egypt (P.T/PED/2/2023/30) on 14 February 2023.
Conflict of interest
The authors have reported no conflicts of interest.
References
Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: A clinical overview. Transl Pediatr. 2020;9(1):S125-S135. doi:10.21037/tp.2020.01.01
Bala I, Tuli R, Dhiman I, Sharma RK, Gautam P. A Study of Ocular Abnormalities in Children with Cerebral Palsy. 2023;(August).
Hosseini P, Kobravi HR, Tahami E, et al. A New Training Protocol Based on Bimanual Playing a Computer Game for Motion-Cognitive Rehabilitation in Children with Spastic Hemiparetic Cerebral Palsy. Iran J Pediatr. 2023;33(5). doi:10.5812/ijp-136889
Gaillard F, Cacioppo M, Bouvier B, et al. Assessment of bimanual performance in 3-D movement analysis: Validation of a new clinical protocol in children with unilateral cerebral palsy. Ann Phys Rehabil Med. 2020;63(5):408-415. doi:10.1016/j.rehab.2019.06.008
Levitt S, Addison A. Treatment of Cerebral Palsy and Motor Delay.; 2018.
Sabir OA, Alshomrani AM, Alqarni WM, Bamusa KA, Johnson EG. Effects of vestibular rehabilitation exercises on children with hearing loss, cerebral palsy, and attention deficit hyperactivity disorder: A systematic review. Curr Pediatr Res. 2022;26(4):1322-1330. doi:10.35841/0971-9032.26.4.1322-1330.Abstract
Van Hecke R, Danneels M, Dhooge I, et al. Vestibular Function in Children with Neurodevelopmental Disorders: A Systematic Review. J Autism Dev Disord. 2019;49(8):3328-3350. doi:10.1007/s10803-019-04059-0
Ravarian A, Mohseni-Bandpei MA, Rahmani N, Sajedi F, Soleimani F. The Effects of Vestibular Stimulations on Neurodevelopment, Growth and Vital Signs of Preterm Infants: A Systematic Review. Int J Pediatr. 2022;10(10):16887-16900. doi:10.22038/IJP.2022.66102.4978
Zanella LW, Valentini NC, Copetti F, Nobre GC. Peabody Developmental Motor Scales - Second Edition (PDMS-2): Reliability, content and construct validity evidence for Brazilian children. Res Dev Disabil. 2021;111(February). doi:10.1016/j.ridd.2021.103871
Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int. 2002;9(3):201-209. doi:10.1002/oti.165
Goyal C, Vardhan V, Naqvi W, Arora S. Effect of virtual reality and haptic feedback on upper extremity function and functional independence in children with hemiplegic cerebral palsy: a research protocol. Pan Afr Med J. 2022;41. doi:10.11604/pamj.2022.41.155.32475
Said RGAEA, Elmonem AMA, Aly MG. Correlation between selective motor control and upper extremity function in children with hemiparesis. Pakistan J Med Heal Sci. 2021;15(6):1709-1712. doi:10.53350/pjmhs211561709
Niazi B, Kalantari M, Azhdar M, Tabatabaee SM, Rezaee M, Daryabor A. The Relationship Between the Fine Motor Skills and Occupational Self-Assessment of Children with Hemiplegic Cerebral Palsy. Middle East J Rehabil Heal Stud. 2022;9(3).
Abd El Wahab M, Hamed NES. Effect of hand-arm bimanual intensive therapy on fine-motor performance in children with hemiplegic cerebral palsy. Egypt J Med Hum Genet. 2015;16(1):55-59. doi:10.1016/j.ejmhg.2014.07.005
te Velde A, Morgan C, Finch-Edmondson M, et al. Neurodevelopmental Therapy for Cerebral Palsy: A Meta-analysis. Pediatrics. 2022;149(6). doi:10.1542/peds.2021-055061
Sailesh KS, Ravikanth M, Kumar AHS. Effect of Vestibular Stimulation on Different Body Systems: A Overview. J Med Sci Heal. 2018;04(02):1-10. doi:10.46347/jmsh.2018.v04i02.001
Kuatsjah E, Khoshnam M, Menon C. Investigation on the effect of noisy galvanic vestibular stimulation on fine motor skills during a visuomotor task in healthy participants. PLoS One. 2019;14(5):1-23. doi:10.1371/journal.pone.0216214
Malawade M, Shaikh TS. Effectiveness of vestibular stimulation training in cerebral palsy. Medico-Legal Updat. 2020;20(2):369-374. doi:10.37506/mlu.v20i2.1132
Elsayed AM, Salem EE, Eldin SMN, Abbass ME. Effect of using adaptive seating equipment on grasping and visual motor integration in children with hemiparetic cerebral palsy: a randomized controlled trial. Bull Fac Phys Ther. 2021;26(1). doi:10.1186/s43161-021-00046-8