Research - (2024) Volume 19, Issue 4
EFFECTS OF PHYSICAL ACTIVITY ON SLEEP QUALITY, INHIBITORY CONTROL, WORKING MEMORY AND COGNITIVE FLEXIBILITY AMONG ADOLESCENT STUDENTS
Dr. Mohammad Hossein Manzari Tavakoli1*, Prof. Masoumeh Shojaei2 and Keyvan Molanorouzi2*Correspondence: Dr. Mohammad Hossein Manzari Tavakoli, Department of Motor Behavior and Sport Psychology, Islamic Azad University, Science and Research Branch Iran, Iran, Email:
Abstract
Background: The objective of this current research is to examine the impacts of a physical activity intervention on sleep quality and executive functions. Specifically, we will examine whether moderate-intensity resistance training has a more beneficial impact on sleep quality and executive functions among a sample of male adolescent than an active control condition.
Method: Male children, aged 12-14 years as participants were enlisted to participate in the ongoing intervention study. Subsequently, they were randomly allocated to either a resistance training or to an active control condition. Participants were under go sleep quality and executive function tests included inhibitory control, working memory and cognitive flexibility at the start of the study, eight weeks following the interventions conclusion, and once more, four weeks later during the follow-up period. The resistance intervention was consist of three 45min group sessions per week, across eight consecutive weeks.
Results: Result showed that compared to an active control condition and compared to the baseline, participating in the resistance training program lead to significant improvements in sleep quality and execution functions over time.
Conclusion: The findings of the present study have the potential to provide guidance to physical educators, parents or to children themselves regarding the question of how they can effectively improve their sleep, physical fitness and cognitive performance, which in turn might have a positive effect on their academic function and their perceived quality of life.
Keywords
Adolescent. Sleep. Physical activity. Cognition. Motor development. Psychomotor
Introduction
Adolescent is a life stage that brain under goes significant development, particularly in the prefrontal cortical regions responsible for executive functioning [1]. Hence, it is crucial to discover ways to improve brain health during this developmental phase to optimize cognitive growth, academic performance, and career success [2]. Moreover, sleep problems among adolescents have become a prevalent concern, drawing attention due to their impact on physical health, mental well-being, and overall academic performance [3]. Epidemiological studies reveal that a significant proportion of adolescents experience sleep-related difficulties. Based on previous studies [4,5], a substantial number of adolescents, ranging from 60% to 70%, report insufficient sleep on school nights. Previous studies have shown that prevalence of risk taking and impulsivity behaviour is high among adolescent [6]. However, sleep and executive functions enhancement can help to decrease these impulsivity among this population [7]. Therefore, it is worth to conduct intervention about patterns of sleep, brain development and behaviour.
The serious health, social and academic consequences of less physical activity life style are generally confirmed. Physical exercise is one of the most common interventions to improve cognitive and sleep quality in adolescents [8,9]. For instance, in a study, Gerber, Feldmeth [10] found that adolescents who have higher physical activity reported significantly higher mental health and fitness scores. Likewise, Lin, Mutz, Clough, and Papageorgiou (2017) showed that higher mental fitness scores were associated with a higher learning, educational and work performance, and with psychological well-being. Therefore, it is necessary to focused on physical activity to enhance sleep quality, cognitive functions and academic achievements in adolescents [11].
Research on physical training aimed at enhancing sleep quality and cognitive functioning has primarily focused on manipulating the quantitative aspects of exercise. The main objective of such studies is to establish a correlation between the amount of physical activity and cognitive performance, commonly known as a dose-response relationship [12]. Inactive lifestyle is associated with a reduction in the cognitive and sleep quality in adolescents [13,14]. However, studies have found that engaging in resistance training and subsequently experiencing increased muscular strength is linked to enhanced cognitive function, improved body composition, and sleep quality [15-17]. Based on the literature, resistance training is not considered for improving the sleep quality and cognition among adolescents in the most studies [16]. Further, in the intervention studies, muscle strength has been less examined, and emphasized are more on the aerobic exercises [18,19].
The executive functions component is related to inhibitory control, working memory, and division of attention. Practicing these functional components may be an important strategy to prevent impulsivity in adolescents [20]. Regard to executive functions, the limited number of studies examining the impact of resistance training on these aspects of cognitive function have yielded inconclusive results [21]. While, research has shown a correlation between resistance training and enhancements in global cognitive performance, balance, and motor skills [15,21], there is few resistance training intervention in adolescent. A recent systematic review has demonstrated that resistance training yielded positive effects on overall cognitive function [22]. Among different exercise modes, resistance training in particular has come to the fore, because it is suggested to stimulate higher order cognition [21,23]. Moreover, based on previous studies, the possible adaptability of the brain and its connection to executive functions and sleep could explain the improvement in cognitive performance following resistance training [24,25]. However, it remains unclear how resistance training should be designed to elicit greater improvements in sleep and cognitive performance specially in adolescents [26].
Higher-order cognition, particularly executive functioning, is linked to various health-related outcomes throughout all stages of life [21, 23,27]. While the evidence is not extensive, the current state of research suggests that resistance training has positive short-term and long-term effects on executive functions [21]. However, there is still a lack of knowledge regarding the effectiveness of specific resistance training programs in various populations and age groups [22, 24,28]. For example, the impact of resistance training on sleep quality and executive function in children and adolescents still remains unclear [21]. The present study will examine how moderate-intensity resistance training impacts on sleep quality and executive functions among adolescents students. We expect that compared to an active control condition, participating in the resistance training program will lead to significant improvements in sleep quality and execution functions over time. The planned research is original because it may show new ways how to impact on cognitive development, sleep quality and vocational attainment in adolescents. This new training program will be helpful for physical education teachers, parents and the adolescents themselves to improve their sleep quality and cognitive function.
Methods
Our study was structured as a randomized controlled trial. Data collection occurred at three time points: baseline, 8 weeks after the interventions completion, and 4 weeks later during the follow-up period.
Participants
Sixty adolescent from the Iranian primary/secondary school were approached and invited to participate in the present study. Children were invited and selected to take part in the current study based on an initial screening process. To be eligible for the study, participants had to meet the following inclusion criteria: (1) being male, (2) age between 10 and 14 years, (3) attended as a students in the Iranian primary or secondary school, (4) having normal results on a Snellen chart test for vision and self-reporting normal hearing abilities. Participants were excluded if they had any psychiatric issues, which were determined through a brief psychiatric interview, (2) neuromuscular, motor and/or sensory disorders and (3) intake of mood- and alertness-altering medications.
The participants were randomly assigned to either the intervention or control group. The average age of the participants was 12.98 years with a standard deviation of 0.44 years. Following this, all participant's parents read and agreed to an informed consent form that had been approved by the local ethics board (Islamic Azad University). Participants completed a series of tests assessing inhibitory control, working memory and cognitive flexibility and sleep quality. The intervention for physical activity involved three 45-minute group sessions per week for a period of 8 consecutive weeks. In contrast, participants in the control group met in small groups twice a week for 30 minutes to exchange informal experiences.
A power analysis was conducted using G*Power 3.1 software, which showed that a minimum of 28 participants were needed to detect a moderate effect size (f = .25) with a significance level of α = .05, power of .80, two groups, three measurements, and a correlation of .50 among repeated measures in repeated measures analysis of variance (rANOVAs).
Physical activity intervention
The resistance training in this study involved a weight lifting training method that was conducted three times per week. Adolescent students were instructed to perform multi-joint resistance training exercises that targeted major muscle groups in the upper and lower body, such as the shoulders, arms, hips, and legs. In each session, they were required to perform three sets of resistance exercises for each major muscle group, with 8 to 12 repetitions at 60-75% of their 1-repeat maximum (1RM). Before the beginning of the session, cycling and stretching constituted the warm-up protocol. The training sessions, which lasted approximately 30 to 45 minutes, were over seen by trained clinical sport scientists.
Control condition
In the control group, participants met twice a week in small groups of six to nine individuals for a period of eight consecutive weeks, with each session lasting 45 minutes. During these sessions, they engaged in group discussions and activities such as listening to music, meal planning, interpreting food labels, and playing darts. Additionally, participants in the control group did not receive any further intervention or have any contact with the study center.
Outcome measures
Sleep quality. The Adolescent Sleep Wake Scale (ASWS) is a self-report tool comprising 28 items designed to assess overall subjective sleep quality. It encompasses five sleep behavior subscale: Going to Bed, Falling Asleep, Maintaining Sleep, Reinitiating Sleep, and Return to Wakefulness. Respondents rate each item on a scale ranging from 1 ("Always") to 6 ("Never"). Both total and subscale scores are calculated as averages of the respondent's answers, with scores ranging from 1 to 6. Higher scores on the ASWS indicate better sleep quality. Psychometric evaluation of the ASWS has confirmed its suitability for use with adolescents (ages 12–18), showing internal consistency values ranging from 0.64 to 0.82 [29].
Inhibitory control. The researchers evaluated children's inhibition by administering a computerized Simon task [30]. During the task, adolescent students were presented with pictures of animals, such as cats and dogs, on a computer screen. They were instructed to press the left key on the keyboard when they saw a cat, regardless of its position on the screen, and to press the right key when they saw a dog, regardless of its position. The task consisted of a block of 14 consistent trials (with dogs on the left) and 14 inconsistent trials (with cats on the right), followed by a block of 24 mixed consistent and inconsistent trials. Each adolescent student then completed 30 consistent and 30 inconsistent trials in the inhibition task. Interstimulus intervals of 1000 ms were used, and trials ended after the participants responded. Accuracy and response times were recorded.
Working memory. To measure working-memory performance, the researchers used the N-back task. This task involved presenting a sequence of letters and numbers [31]. To assess working-memory performance, adolescent students were asked to determine whether the current numbers/letters matched the numbers/letters presented one or two trials earlier in the series. They were required to respond by pressing a button for each trial to indicate whether the match was correct or not. The task consisted of two levels of difficulty, namely one-back and two-back. The stimuli were blue letters with a vertical visual angle of 0.7°, presented on a light black background. Each number/letter in the sequence was displayed for 1000 ms, with a response window of 2,500 ms provided. The inter-stimulus interval was 2,000 ms. Targets, which were letters matching the letter presented N trials earlier, occurred with a probability of 25% in each block. Scores were based on the mean reaction time for correct responses. Test-retest reliability of the task was confirmed, with a correlation coefficient of r = .79 for one-back and r = .72 for two-back.
Cognitive flexibility. To evaluate cognitive flexibility, the researchers used the Wisconsin Card Sorting Task. This test measured cognitive flexibility by examining changes in accuracy and/or response time when the correct way of responding was modified (known as the switch cost). During the task, a series of stimulus cards were presented to the adolescent, who was instructed to match the cards without being told how to match them. The adolescent was provided with feedback indicating whether their match was correct or incorrect. The task was carried out with paper card placed on a desk, with the experimenter on one side and the adolescent on the other. The test typically takes 12-20 minutes to complete and generates various psychometric scores, including trials, errors, and perseverative errors [32].
Statistical analysis
To compare the two groups, we executed a series of independent t-tests on variables encompassing age, educational level, weight, and BMI. Subsequently, a sequence of repeated measures analyses of variance (rANOVAs) was employed, considering Time (baseline, post-intervention, follow-up) and Group (intervention vs. control) as factors, along with the Time by Group interaction. Sleep quality, inhibitory control, working memory, and cognitive flexibility served as the dependent variables in these analyses. To address deviations from sphericity, Greenhouse-Geisser corrected degrees of freedom were utilized in the rANOVAs, with the original degrees of freedom and the relevant Greenhouse-Geisser epsilon value (ε) reported. Assessing the magnitude of changes within each group from baseline to post-intervention and follow-up involved dependent sample t-tests, while independent sample t-tests were conducted to scrutinize differences between the two groups at baseline, post-intervention, and follow-up. A significance level of p ≤ .05 was established, and all statistical analyses were executed using SPSS® 25.0 (IBM Corporation, Armonk, N.Y., USA) for Apple McIntosh ®.
Results
All 60 students successfully completed the baseline, post-intervention, and follow-up data assessments. Among the 30 students allocated to the intervention group, each actively participated in the entirety of the planned 12 resistance training sessions. Meanwhile, the remaining 30 students assigned to the control group engaged in the designated control sessions. Sociodemographic characteristics of participants in both the intervention and control groups are outlined in Table 1. Furthermore, inferential statistical analyses were conducted to ascertain if there were disparities in baseline sociodemographic variables between the two groups. Descriptive statistics detailing outcome variables (inhibitory control, working memory, and cognitive flexibility) are presented separately for both the intervention and control groups, as well as across the three measurement occasions (baseline, post-intervention, and follow-up) in Table 2. The findings from the rANOVAs, which were employed to investigate the divergent development of assessed outcome variables between the two groups, are also elucidated in Table 2.
| Statistics | Group | ||
|---|---|---|---|
| Control | Physical activity | Dimension | |
| t(58) = 0.93, p = .38
t(58) = 0.92, p = .29 t(58) = 1.02, p = .30 c2(N = 40, df = 1) = 0.11, p = 0.74 |
30 12.83 (0.61) 12-14 48.29 (2.01) 17.32 (0.39) 29/31 |
30 12.13 (0.28) 12-14 48.32 (1.88) 17.98 (0.45) 32/28 |
N Age (years): M (SD) Age range (years) Weight (kg) M (SD) Body mass index (kg/m2): M (SD) Highest educational level (primary school/secondary school): n |
| Groups | Factors | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Group | Time | Time x Group interaction | ||||
| N | 30 | 30 | ||||||
| M (SD) | M (SD) | F | ηp2 | F | ηp2 | F | ηp2 | |
| Sleep quality | 4.78* | .17 | 31.28 ** | .43 | 7.26** | .42 | ||
| Baseline | 3.45 (0.24) | 3.54 (0.30) | ||||||
| Post-intervention | 2.22 (0.02) | 3.32 (0.42) | ||||||
| Follow-up | 2.70 (0.51) | 3.30 (0.48) | ||||||
| Inhibitory control Baseline Post-intervention Follow-up |
3.77 (0.84) 5.71 (2.02) 4.54 (0.91) |
3.62 (0.72) 3.25 (0.64) 3.43 (0.88) |
5.42* | .15 | 26.43 ** | .45 | 21.23** | .58 |
| Working memory | 17.21*** | .25 | 20.29** | .61 | 16.22 ** | .66 | ||
| Baseline | 2.65 (0.77) | 2.15 (1.09) | ||||||
| Post-intervention | 5.15 (1.06) | 2.26 (0.82) | ||||||
| Follow-up | 5.05 (0.35) | 2.07 (0.81) | ||||||
| Cognitive flexibility | 4.16* | .11 | 25.31** | .56 | 27.45** | .66 | ||
| Baseline | 43.01 (7.98) | 47.40 (3.34) | ||||||
| Post-intervention | 21.40 (2.32) | 45.82 (5.15) | ||||||
| Follow-up | 22.15 (3.18) | 42.40 (4.75) | ||||||
Sample characteristics
Table 1 provides information about the sociodemographic background of the participants, separately for the intervention and the control group. The inferential statistics show that the two groups did not significantly differ with respect to age, educational level, weight and BMI (Table 1).
Between-group differences across time
Means and standard deviations for all outcomes at baseline, post-intervention and follow-up are provided in (Table 2), separately for the intervention and control conditions.
Sleep quality: The repeated-measures analyses of variance (rANOVAs) demonstrated a notable decrease in sleep problems across the entire sample from the baseline to the conclusion of the study and the subsequent follow-up. This noteworthy effect was primarily attributed to a substantial enhancement in sleep quality within the resistance training group, as indicated by the significant Time by Group interaction. The calculation of effect sizes (Cohen's d) revealed a considerable reduction in sleep problems within the resistance group from baseline to post-intervention (large effect: d =1.61) and from baseline to follow-up (medium effect: d = 0.92). In contrast, sleep problems in the control group exhibited minimal change from baseline to post-intervention (d=0.15) and from baseline to follow-up (d = 0.17). Effect size computations comparing group means indicated comparable sleep quality scores at baseline (d = 0.08), while a substantial difference between the intervention and control groups emerged at post-intervention (large effect: d = 1.94) and follow-up (medium effect: d = 0.98). The statistical significance of these findings is detailed in Table 2.
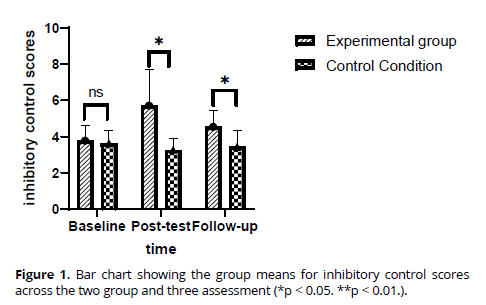
Inhibitory control. In the domain of inhibitory control, the repeated-measures ANOVAs (rANOVAs) unveiled a notable increase in scores from the initial assessment to the conclusion of the study and the subsequent follow-up period across the entire participant cohort (refer to figure 1). This noteworthy effect primarily stemmed from a marked enhancement in inhibitory control within the resistance group, substantiated by the significant interaction between Time and Group. Evaluation of effect sizes (Cohen's d) illustrated a considerable rise in inhibitory control scores within the resistance group, both post-intervention (substantial effect: d =1.23) and during the follow-up (moderate effect: d = 0.60), compared to baseline. Conversely, inhibitory control scores within the control group exhibited minimal alterations from baseline to post-intervention (d = 0.05) and follow-up (d = 0.07). Effect size calculations for group means indicated comparable inhibitory control scores at baseline (d = 0.05), while noteworthy differences between the intervention and control groups emerged at post-intervention (substantial effect: d = 1.82) and follow-up (moderate effect: d = 0.81) (Figure 1) The statistical significance of these outcomes is detailed in Table 2.
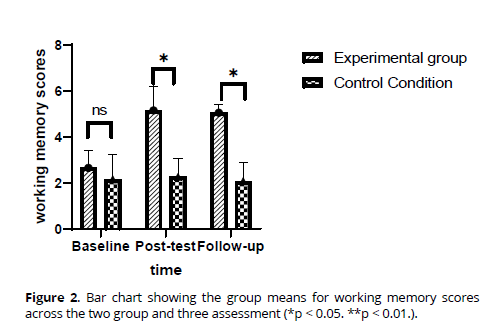
Working memory. The rANOVAs indicated that working memory scores increased from baseline to intervention completion and follow-up in the sample as a whole, with a significant Time effect as shown in Table 2. The improvement in working memory was primarily attributed to the intervention group (Figure 2), as indicated by the significant Time x Group effect, while working memory scores remained almost unchanged from baseline to follow-up in the control group. Effect size calculations showed that working memory scores in the intervention group increased substantially from baseline to post-intervention (large effect: d = 1.93) and from baseline to follow-up (large effect: d = 1.32). However, working memory scores in the control group remained nearly unchanged from baseline to post-intervention (d = 0.13) and from baseline to follow-up (d = 0.09). Effect size calculations comparing the means of the intervention and control groups showed that working memory scores were similar at baseline (d = 0.19), but differences became more pronounced at post-intervention (large effect: d = 1.32) and follow-up (large effect: d = 1.09). Statistical significance for these findings is presented in Table 2.
Cognitive flexibility. The rANOVAs revealed that cognitive flexibility error decreased across the sample as a whole from baseline to post-intervention and follow-up, as shown in Table 2. However, the significant Time effect was primarily due to a reduction in cognitive flexibility error in the intervention group (Figure 3), while the control group's scores remained at a similar level from baseline to follow-up. Effect size calculations indicated that cognitive flexibility error in the intervention group decreased substantially from baseline to post-intervention (large effect: d = 2.06) and from baseline to follow-up (large effect: d = 1.63). In contrast, cognitive flexibility error in the control group remained almost the same from baseline to post-intervention (d = 0.06) and from baseline to follow-up (small effect: d = 0.21). Comparing the means of the intervention and control groups, both groups had similar scores at baseline (large effect: d = .62), but at post-intervention (large effect: d = 1.78) and follow-up (large effect: d = 0.90), the control group reported higher error than the intervention group. These results are statistically significant, as presented in Table 2.
Discussion
The main results of this study indicate that participating in an eight-week course of resistance training improved sleep quality and executive functions in adolescents compared to an active control condition. Crucially, the improvements in sleep quality and executive functions observed in the resistance training group persisted for four weeks after the completion of the intervention program. These findings are valuable addition to the current literature, as they demonstrate the successful application of resistance training in improving sleep quality and executive functions among adolescent students.
There is huge evidence that showed physical activity appears to be a promising intervention in the sleep quality and cognitive enhancement [33-35]. The current study provides evidence that resistance training can increase sleep quality and executive functions among adolescent students. This finding is consistent with Esteban-Cornejo, Tejero-Gonzalez [34] study showing that physical activity led to a increase in cognitive functions in adolescents. Moreover, our results were in line with Wang and Boros [36] study that showed physical activity can improve sleep quality.
Previous studies have shown that environmental stimuli such as physical activity have positive effects on cognitive development during childhood [37]. Physical activity leads to increased synapses and memory formation in the brain. In a study, Gomes da Silva and Arida [38] showed that physical activity intervention can increase hippocampal Volume. Therefore, physical activity can play a key role in promoting cognitive function and academic achievement. In this line and in a review study based on sixteen studies, Sibley and Etnier [39] showed that there is a positive and significant relationship between physical activity and learning and academic score in children. Furthermore, structural and functional changes in the brain following physical activity have been confirmed [40]. Moreover, enhancement of basic motor skills can improve cognitive and brain functions. As a result, physical activity can strengthen relationships with peers, parents and teachers, which is an important factor in mental health and cognitive function improvement [41].
The results of present study showed that resistance training can improve inhibitory control in adolescents student. This finding is consistent with the findings of Ludyga, Gerber [42], Hillman, Erickson [43] and Khan and Hillman [44]. Our finding was consist with Ludyga et al. (2018) study who found that 8 physical activity based intervention can enhances adolescents' inhibitory control. There is evidence that showed higher inhibitory control is associated with lower impulsive behaviors [45]. Impulsive behaviors are common among adolescents. Impulsivity is presented as a multiple structure that is associated with cognitive impairment such as little attention and reduced control and inhibition. As a result, special attention to impulsivity can be very important in improving mental health. In this regard, Carragher, Teesson [46] found that to reduce antisocial behaviors in adolescents, interventions that target impulsivity and inhibitory control should be used. There is relationship between physical health, cognitive function and academic performance in children and adolescents. Findings have been shown that physical activity can improve academic performance [43]. In addition, the results of the present study are consistent with the results of Rasberry, Lee [47] review. In this regard, physical activity can improve self-esteem and self-control. More self-esteem is associated with more involvement in the school and a greater willingness to learn. Moreover, physical activity in the school environment is associated with higher discipline, creativity and motivation. As a result, physical activity have many positive effects on social, cognitive and academic performance.
These findings are consistent with previous research indicating that physical activity and exercise have positive effects on sleep quality and cognitive function. One plausible interpretation of this discovery is that engagement in physical activity and exercise might elevate the levels of brain-derived neurotrophic factor (BDNF), a neurotrophic known for promoting neurogenesis. This elevation in BDNF levels could potentially contribute to an enhancement in sleep quality [48] and working memory functioning [49,50]. Moreover, existing evidence suggests that resistance training could influence cognitive function through neurobiological pathways. Interventions based on physical activity, including resistance training, have demonstrated an ability to increase both gray and white matters Volume in the frontal cortex [51,52]. Consequently, the release of neurotrophic factors, such as BDNF and insulin-like growth factor-1 (IGF-1), is theorized to result in an augmented Volume of the temporal lobe and prefrontal cortex [53] affecting emotion regulation, learning and memory capacity. This proposition may elucidate the observed enhancements in sleep quality and working memory performance within the resistance exercise training group in this current study.
Despite the innovative nature of these findings, several limitations warrant caution against broad generalizations. Primarily, the study is constrained by a relatively small sample size, a notable limitation. However, it's noteworthy that statistical calculations relied on effect sizes, which remain unaffected by sample size. Secondly, no biomarkers, such as cortisol (indicative of perceived stress) or BDNF (indicative of neural plasticity), were measured. Thirdly, the possibility exists that unmeasured variables could have influenced two or more factors either in the same or opposing directions, potentially impacting the observed pattern of results. Lastly, the study exclusively involved male students, leaving uncertainties about the applicability of these findings to female students and the extent of such applicability.
Conclusion
The findings of this research suggest that resistance training can enhance sleep quality, inhibitory control, working memory, and cognitive flexibility. Future studies could compare the effects of resistance training with other forms of exercise training, such as aerobic and strength training, on both sleep quality and cognitive function. However, there is limited evidence currently available that suggests the superiority of one form of exercise training over another [54].
Ethics approval and consent to participate
The informed consent was obtained from all the parents or legal guardians for the study. The Review Board of the Islamic Azad University, Science And Research Branch (Tehran, Iran) approved the study, which was performed in accordance with the ethical principles laid down in the seventh and current edition (2013) of the Declaration of Helsinki (Trial Registration Number: IAUSR1400342).
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to the fact that data are identified but are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Authors’ contributions
Mohammad Hossein Manzari Tavakoli, Masoumeh Shojaei and Kyvan Molanorouzi participated in the design of the study, carried out the data, analyzed the data, interpreted the results, and wrote the Manuscript. All authors read and approved the final Manuscript.
Conflict of Interest statement
The authors do not have any interests that might be interpreted as influencing the research, and ethical standards were followed in the conduct of the study. All authors declare no conflict of interests.
Acknowledgment
We wish to express our gratitude to all participants and their parents for assist in data collection.
References
Blakemore SJ, den Ouden H, Choudhury S, et al. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007 Jun;2(2):130-9.
Kleibeuker SW, De Dreu CK, Crone EA. The development of creative cognition across adolescence: distinct trajectories for insight and divergent thinking. Developmental science. 2013;16(1):2-12.
Wolfson AR, Carskadon MA. Understanding adolescent's sleep patterns and school performance: a critical appraisal. Sleep medicine reviews. 2003;7(6):491-506.
Owens J, Group ASW, Adolescence Co, et al. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921-e932.
Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep medicine reviews. 2014;18(1):75-87.
Romer D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Developmental psychobiology. 2010 Apr;52(3):263-76.
Nordvall O, Neely AS, Jonsson B. Self-Reported Impulsivity and its Relation to Executive Functions in Interned Youth. Psychiatry, psychology, and law : an interdisciplinary journal of the Australian and New Zealand Association of Psychiatry, Psychology and Law. 2017;24(6):910-922.
Esteban-Cornejo, Tejero-Gonzalez CM, Sallis JF, et al. Physical activity and cognition in adolescents: A systematic review. Journal of science medicine in sport 2015;18(5):534-539.
Rosa CC, Tebar WR, Oliveira CBS, et al. Effect of different sports practice on sleep quality and quality of life in children and adolescents: randomized clinical trial. Sports Medicine-Open. 2021;7(1):1-10.
Gerber, Feldmeth, Lang, et al. The relationship between mental toughness, stress, and burnout among adolescents: a longitudinal study with Swiss vocational students. Psychological reports. 2015;117(3):703-723.
Lin Y, Mutz J, Clough PJ, et al. Mental toughness and individual differences in learning, educational and work performance, psychological well-being, and personality: A systematic review. Frontiers in psychology. 2017;8:1345.
Forte R, Boreham CA, Leite JC, et al. Enhancing cognitive functioning in the elderly: multicomponent vs resistance training. Clinical interventions in aging. 2013;8:19.
Boreham C, Riddoch C. The physical activity, fitness and health of children. Journal of sports sciences 2001;19(12):915-929.
Lang C, Brand S, Feldmeth AK, et al. Increased self-reported and objectively assessed physical activity predict sleep quality among adolescents. Physiology behavior. 2013;120:46-53.
Orr R, Raymond J, Singh MF. Efficacy of progressive resistance training on balance performance in older adults. Sports Medicine. 2008;38(4):317-343.
Costigan SA, Eather N, Plotnikoff RC, et al. High-intensity interval training on cognitive and mental health in adolescents. Medicine Science in Sports Exercise 2016;48(10):1985-1993.
Whitworth JW, Nosrat S, SantaBarbara NJ, et al. High intensity resistance training improves sleep quality and anxiety in individuals who screen positive for posttraumatic stress disorder: A randomized controlled feasibility trial. Mental Health Physical Activity. 2019;16:43-49.
Jehu DA, Paquet N, Lajoie Y. Balance and mobility training with or without simultaneous cognitive training reduces attention demand but does not improve obstacle clearance in older adults. Motor control. 2017: 20 (XX):1-20.
Alfieri FM, Riberto M, Gatz LS, et al. Comparison of multisensory and strength training for postural control in the elderly. Clinical interventions in aging. 2012; 7:119.
Yu Y, Mo PK-H, Zhang J, et al. Impulsivity, Self-control, Interpersonal Influences, and Maladaptive Cognitions as Factors of Internet Gaming Disorder Among Adolescents in China: Cross-sectional Mediation Study. Journal of Medical Internet Research. 2021; 23(10):e26810.
Soga K, Masaki H, Gerber M, et al. Acute and Long-term Effects of Resistance Training on Executive Function. Journal of Cognitive Enhancement. 2018:1-8.
Li Z, Peng X, Xiang W, et al. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clinical and Experimental Research. 2018:1-15.
Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Jama. 2002;288(12):1475-1483.
Ludyga S, Gerber M, Brand S, et al. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta‐analysis. Psychophysiology. 2016;53(11):1611-1626.
Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Archives of internal medicine. 2012;172 (8):666-668.
Pliatsikas C, Veríssimo J, Babcock L, et al. Author accepted manuscript: Working memory in older adults declines with age, but is modulated by sex and education. Quarterly Journal of Experimental Psychology. 2018:1747021818791994.
Crone EA. Executive functions in adolescence: inferences from brain and behavior. Developmental science. 2009;12(6):825-830.
Norouzi E, Vaezmosavi M, Gerber M, et al. Dual-task training on cognition and resistance training improved both balance and working memory in older people. The Physician and sportsmedicine. 2019:1-8.
LeBourgeois MK, Giannotti F, Cortesi F, et al. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(Supplement_1):257-265.
Lee K, Bull R, Ho RMJCd. Developmental changes in executive functioning. Child development 2013;84(6):1933-1953.
Ruiz, Carrillo-Sanchez K, Gomez-Lopez N, et al. Working memory performance in young adults is associated to the AATn polymorphism of the CNR1 gene. Behav Brain Res. 2013 Jan 1;236(1):62-6.
Somsen RJ. The development of attention regulation in the Wisconsin Card Sorting Task. Developmental science 2007;10(5):664-680.
Bidonde, Boden C, Busch AJ, et al. Dance for adults with fibromyalgia—what do we know about It? Protocol for a scoping review. JMIR research protocols. 2017;6(2).
Esteban-Cornejo, Tejero-Gonzalez, Sallis, et al. Physical activity and cognition in adolescents: A systematic review. Journal of science and medicine in sport. 2015;18(5):534-539.
Kredlow MA, Capozzoli MC, Hearon BA, et al. The effects of physical activity on sleep: a meta-analytic review. Journal of behavioral medicine. 2015; 38:427-449.
Wang F, Boros S. The effect of physical activity on sleep quality: a systematic review. European Journal of Physiotherapy. 2021;23 (1):11-18.
Goodway JD, Ozmun JC, Gallahue DL. Understanding motor development: Infants, children, adolescents, adults. Jones & Bartlett Learning; 2019.
Gomes da Silva S, Arida RM. Physical activity and brain development. Expert review of neurotherapeutics 2015;15(9):1041-1051.
Sibley, Etnier. The relationship between physical activity and cognition in children: a meta-analysis. Pediatric Exercise Science. 2003;15(3):243-256.
Blakemore. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007.
Hooke MC, Rodgers C, Taylor O, et al. Physical activity, the childhood cancer symptom cluster-leukemia, and cognitive function: A longitudinal mediation analysis. Cancer nursing. 2018;41(6):434.
Ludyga, Gerber, Herrmann, et al. Chronic effects of exercise implemented during school-break time on neurophysiological indices of inhibitory control in adolescents. Trends in Neuroscience and Education. 2018;10:1-7.
Hillman, Erickson, Kramer. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews neuroscience. 2008;9(1):58-65.
Khan, Hillman. The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. J Brain research. 2014;26(2):138-146.
Pauli-Pott U, Albayrak Ö, Hebebrand J, et al. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: dependence on age and inhibitory control component. Child Neuropsychology 2010;16(6):592-603.
Carragher, Teesson, Sunderland, et al. The structure of adolescent psychopathology: a symptom-level analysis. Psychological Medicine. 2016;46(5):981-994.
Rasberry, Lee, Robin, et al. The association between school-based physical activity, including physical education, and academic performance: a systematic review of the literature. Preventive Medicine. 2011;52:S10-S20.
Norouzi E, Mohammadi R, Fadaei R, et al. A systematic review and meta-analysis on the levels of brain-derived neurotrophic factor in insomnia patients with and without comorbid depression. Biological Rhythm Research. 2023:1-12.
Sosa MD, Nunez-Nagy S, Gallego-Izquierdo T, et al. Effectiveness of therapeutic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. BioMed research international. 2017;2017.
Etnier JL, Nowell PM, Landers DM, et al. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain research reviews. 2006;52(1):119-130.
Rathore A, Lom B. The effects of chronic and acute physical activity on working memory performance in healthy participants: a systematic review with meta-analysis of randomized controlled trials. Systematic reviews. 2017;6(1):124.
Rehfeld K, Muller P, Aye N, et al. Dancing or Fitness Sport? The Effects of Two Training Programs on Hippocampal Plasticity and Balance Abilities in Healthy Seniors. Front Hum Neurosci. 2017; 11:305.
Padilla C, Pérez L, Andrés P. Chronic exercise keeps working memory and inhibitory capacities fit. Frontiers in behavioral neuroscience. 2014; 8:49.
Macfarlane G, Kronisch C, Dean L, et al. EULAR revised recommendations for the management of fibromyalgia. Annals of the rheumatic diseases. 2016:annrheumdis-2016-209724.