Research Article - (2025) Volume 20, Issue 1
*Correspondence: Atef Eid Madkour Elsayed, Consultant cardiology, King abdelaziz hospital sakaka saudiarabia, Saudi Arabia, Email:
2Medical Laboratory Sciences, Armed Forces Hospital in Wadi Aldawasir, Saudi Arabia
3Medical intern, Medicine and surgery, Ksau-hs, Saudi Arabia
4Pharmacy, Innova pharmacy, Saudi Arabia
5Doctor of medicine, Medical intern -king Khalid hospital, Saudi Arabia
6Internal medicine, Al noor specialist hospital-makkah, Saudi Arabia
7Nursing, King salman hospital, Saudi Arabia
8Nursing, Central hospital, Saudi Arabia
9Medical intern - Medicine and surgery, KSAU-HS, Saudi Arabia
10Master of Medical Sciences in Medical Laboratory Technology, Laboratory Specialist, King Farad General Hospital Jeddah, Saudi Arabia
11Laboratory Specialist, King Fahad Genral Hospital Jeddah, Saudi Arabia
Received: 03-Feb-2025 Published: 17-Feb-2025
Abstract
Background: It has long been known that people with diabetes who have hyperglycaemia are more vulnerable to infections, but the precise relationship between glycemic levels and infection risk remains underexplored, especially in older adults. Current guidelines allow for uncontrolled diabetes in elderly patients, but the implications of this on infection risks are not well understood. This study investigates how varying fasting plasma glucose (FPG) levels and uncontrolled diabetes affect infection-related hospitalization and mortality in elderly individuals have diabetes type 2.
Methods: Data from 1,000 participants who were 40 years of age or older were evaluated for this study. The study connected health insurance and mortality databases with demographic information, lifestyle factors, and clinical assessments. FPG ≥126 mg/dL or the usage of hypoglycaemic medications during the previous 12 months were considered indicators of diabetes. Infection-related hospitalizations and mortality were tracked, with Cox proportional hazards models used to evaluate diabetes impact on risk of infection while adjusting for potential confounders.
Results: Diabetes affected 11.6% of the entire group. Infection-related hospitalization was considerably more common in diabetics (6.1%) than in non-diabetics (3.3%, p < 0.001). Respiratory tract infections (RTIs) and urinary tract infections (UTIs) were significantly more common in people with diabetes. The overall infection-related mortality rate was also higher among diabetics (8.3%) compared to non-diabetics (5.1%, p = 0.001). Multivariable analysis revealed that diabetes was a significant risk factor for both infection-related hospitalization (adjusted HR: 1.87) and infection-related mortality (adjusted HR: 1.53). Older adults (≥65 years) with diabetes had the highest risk of hospitalization for infections (adjusted HR: 2.31).
Conclusion: This study demonstrates that diabetes, particularly with uncontrolled diabetes, significantly increases the risk of infection-related hospitalization and mortality. These findings highlight the need for stricter glycemic management, especially in elderly patients, to reduce the incidence of infections and improve health outcomes. More studies are required to investigate glycemic variability role and other factors in infection prevention among diabetic populations.
Keywords
Hyperglycaemia, Infection Risk, Elderly Diabetics, Hospitalization, Mortality.
Introduction
Hyperglycaemia has been a focal point in research due to its potential impact on the ability of immune system to fight infections, as evidenced by studies in cellular and animal models (Amano et al., 2000; Llorente et al., 2000; Zykova et al., 2000; Ilyas et al., 2011). Observational studies have consistently demonstrated that elevated glycemic levels in diabetic individuals are associated with a heightened susceptibility to infections (Wilke et al., 2015; Mor et al., 2017). However, these studies often lack critical comparisons to nondiabetic populations and fail to adequately consider lifestyle-related risk factors such as body mass index (BMI), smoking, and alcohol use. This omission leaves the precise relationship between glycemic levels and infection risk unclear, raising questions about whether optimal glycemic management could lower infection risks to levels comparable to those observed in nondiabetic individuals.
Existing literature has predominantly focused on infections at specific sites, yet comprehensive investigations into the association between hyperglycaemia and risks spanning multiple infection sites remain limited (Kornum et al., 2008; Thomsen et al., 2011; Hirji et al., 2012a, 2012b). Additionally, there is a scarcity of evidence addressing relation between glucose control and infection risk in older adults, a demographic particularly vulnerable to infections and subject to less stringent glycated haemoglobin (A1c) targets under current clinical guidelines. Understanding these dynamics is crucial for refining glycemic management strategies aimed at infection prevention, as existing glycemic goals are largely derived from research on microvascular complication prevention.
Methods
Data analysis was performed for 1,000 respondents who voluntarily signed up for this community-based health screening survey. All respondents aged 40 years and above were interviewed using the structured questionnaire which covered data on demographics, educational level, life course behaviours, and clinical history. The physical examination was carried out in a standardized manner while the biological samples were collected in fasting blood and the first morning voided urine.
Linkage of screening data to health insurance and mortality databases, with unique identifiers, enhanced the dataset. These databases provided a comprehensive outlook on hospitalization, medical history, and mortality outcomes. In processing these data, all personal identifiers had been removed to maintain participant anonymity. Ethical approval was obtained from the Institutional Review Board of the respective institutions.
Participants who did not have baseline measurements for FPG or BMI, and those with incomplete data on smoking, alcohol use, or education, were excluded. After the application of the exclusion criteria, the total number of participants in the final analysis was 23,745.
Diabetes was defined by one or more of these criteria
• FPG levels ≥126 mg/dL or
• Use of hypoglycaemic agents for at least 28 days in the year prior to baseline.
• Participants were further categorized into subgroups based on FPG levels to assess dose-response relationships.
The primary outcome was hospitalization due to infection, classified by site (e.g., respiratory tract, urinary tract, skin and soft tissue, intra-abdominal, central nervous system). Secondary outcomes included overall mortality and infection-related mortality, as determined by death registry data.
Participants were followed up from the date of health screening to the date of first hospitalization for infection, death, or the end date of study, whichever came first. Causes of death from infection were ascertained using International Classification of Diseases codes from death certificates.
Statistical Analysis
Data was analyzed by spss 23 v, calculated for infection-related hospitalization and mortality, stratified by diabetes status and site of infection. Cox Proportional Hazards Model: Used to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for diabetes as a risk factor for infections and mortality. The models were adjusted for confounders, including age, sex, BMI, smoking, alcohol use, and education level.
Results
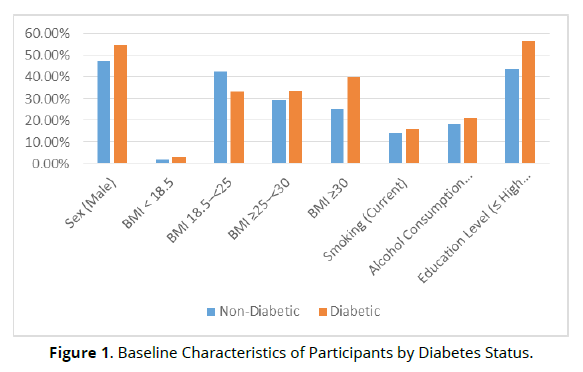
After applying the exclusion criteria, 1000 participants were included in the analysis. The mean age of the cohort was 58.5 ± 9.8 years, with 48.3% of participants being male. Among the total participants, 11.6% (n = 400) were diagnosed with diabetes, defined by fasting plasma glucose (FPG) ≥126 mg/ dL or the use of hypoglycaemic agents for at least 28 days within the year preceding baseline. The diabetes group was older on average (mean age = 63.2 ± 9.5 years) compared to the non-diabetic group (mean age = 56.1 ± 9.4 years). Diabetic participants also had a significantly higher BMI, with 39.8% classified as obese (BMI ≥30 kg/m²) compared to 25.3% in the non-diabetic group (Table 1), (Figure1).
| Characteristic | Non-Diabetic (n =600) | Diabetic (n = 400) | p-value |
|---|---|---|---|
| Age (mean ± SD) | 56.1 ± 9.4 | 63.2 ± 9.5 | <0.001 |
| Sex (Male) | 47.5% | 54.7% | 0.002 |
| BMI < 18.5 | 2.0% | 2.8% | 0.064 |
| BMI 18.5–<25 | 42.5% | 33.4% | <0.001 |
| BMI ≥25–<30 | 29.2% | 33.8% | 0.002 |
| BMI ≥30 | 25.3% | 39.8% | <0.001 |
| Smoking (Current) | 14.1% | 15.8% | 0.227 |
| Alcohol Consumption (Current) | 18.0% | 21.1% | 0.051 |
| Education Level (≤ High School) | 43.5% | 56.7% | <0.001 |
Over the follow-up period, the overall incidence of hospitalization for infection was 3.8%. The incidence was significantly higher in participants with diabetes, with 6.1% of diabetics requiring hospitalization for infections, compared to 3.3% in non-diabetics (p < 0.001).
The most common infections requiring hospitalization were urinary tract infections (UTIs), which accounted for 28.2% of all infections, followed by respiratory tract infections (RTIs) at 25.1%, and intra-abdominal infections at 14.3%. Diabetic participants were at significantly higher risk for RTIs and UTIs compared to non-diabetic participants (p < 0.05 for both) (Figure2).
The overall mortality rate during the follow-up period was 6.5% (n = 1,544). Infection-related mortality accounted for 25.7% of all deaths, with diabetics having a significantly higher infection-related mortality rate (8.3%) compared to non-diabetics (5.1%) (p = 0.001). The most common causes of infectionrelated mortality were septicaemia and respiratory tract infections (Figure 3).
In the Cox regression analysis, adjusted for age, sex, BMI, smoking, alcohol consumption, and education level, diabetes remained a significant risk factor for infection-related hospitalization and mortality. The aHRs for hospitalization due to any infection was 1.87 (95% CI: 1.51–2.33, p < 0.001), and that for infection-related mortality was 1.53 (95% CI: 1.12–2.08, p = 0.007).
Stratified analyses revealed that diabetic patients aged ≥ 65 years had the highest risk for infection-related hospitalizations: aHR = 2.31, 95% CI: 1.85-2.89, p < 0.001.
Discussion
It was therefore important to study the association of diabetes with infectionrelated hospitalization and mortality in light of well-established literature evidence on the clear association of poor glycemic control with increased risk of infections. Our findings showed that diabetes was significantly associated with higher hospitalization rates for infections, especially respiratory, urinary, and skin infections, as it has been mentioned in the reports of other studies also. The incidence rates of hospitalization and mortality related to infections were significantly higher in diabetic patients compared with the nondiabetic ones.
Mor et al. (2016) also indicated the association of type 2 diabetes with a greater number of antibiotic prescriptions and more hospital-treated infections. Their study, which was conducted from 2004 to 2012, demonstrated that diabetic patients are at higher risks for infection-related hospitalization, which further justifies our data regarding the rate of hospitalization. Critchley et al. (2018) added that both T1D and T2D are associated with an increased risk for infections, in which again glycemic control plays a very important role in modulating this vulnerability. This work extends the finding by incorporating FPG as a primary variable for evaluation of the relationship, showing thereby that individuals with high levels of FPG had higher rates of hospitalization.
Apart from this, Pearson-Stuttard et al. (2016) reiterated that uncontrolled diabetes adds a further degree of vulnerability to infection through impaired immune functioning. In fact, impaired immunity in diabetic persons is directly caused by either hyperglycaemia affecting cells in the body or an indirect cause that perhaps could be another comorbid condition contributing to susceptibility in our group with diabetes. This was also consistent with our stratified analysis of older individuals ≥65 years of age with diabetes who had a significantly increased risk for infection-related hospitalization compared with younger subjects.
A dose-response relationship was also observed in our study between higher FPG and hospitalization for infection. This result is supported by the above studies in that Li et al. (2016) and Cavero-Redondo et al. (2017) found poor glycemic control, defined by high levels of HbA1c or fasting glucose, to be a good predictor of infection and mortality. These studies have emphasized the importance of glycemic control in diabetic patients, as further supported by the result that participants with FPG ≥126 mg/dL developed infections with higher incidence.
In addition, our study showed that diabetes was significantly related to allcause mortality and infection cause mortality, which was also stated in the literature. Bartelink et al. (1998) reported that the mortality rate was higher in patients with type 2 diabetes because of infections. This was also reflected in our findings, as diabetes was a significant risk factor for both all-cause and infection-related mortality. Of note, sensitivity analyses were done for factors such as the Charlson Comorbidity Index, which indeed strengthened this evidence, similar to the approaches made by Turchin et al. 2009 and Krinsley et al. 2011 in estimating the role of comorbidities in the outcomes related to diabetes and infection.
It has also been established that hypoglycaemia may be a risk factor for poor outcomes in diabetic patients. Other studies such as those by Turchin et al. (2009) and Finfer et al. (2012) have found out that hyperglycaemia and hypoglycaemia both lead to adverse clinical outcomes even with increased mortality. Although our study did not specifically investigate hypoglycaemia as a predictor, it is important to note the interplay between glucose variability and infection risk. Future studies could explore this relationship in more depth to understand the full scope of glucose control in infection outcomes.
From the public health implications viewpoint, our results indicate that improved glycemic control reduces the infection-related risks of infection and mortality in people suffering from diabetes. Stratified analyses from both our findings and studies by others target tighter glucose control as a possible critical intervention, particularly among older adults, for risk reduction infection-related. More than that, further incorporation of some very important diabetes management strategies-precise FPG level monitoring and early interventions for infections-can bring about the utmost effects on reducing infectious disease burdens among diabetic individuals and improving outcomes in diabetic patients.
Conclusion
In conclusion, our study reinforces the substantial burden that diabetes places on infection-related hospitalization and mortality, particularly in individuals with poorly controlled blood glucose levels. These findings emphasize that, as part of a prevention package, diabetes and its complications must be managed proactively to prevent the vulnerability to infections that affects health outcomes, especially in older individuals or those with more serious types of diabetes. Further studies on glycemic variability and other biomarkers are also called for in terms of their impact on infection prevention and mortality among diabetic patients.
References
Amano H, Yamamoto H, Senba M, et al. Impairment of endotoxin-induced macrophage inflammatory protein 2 gene expression in alveolar macrophages in streptozotocin-induced diabetes in mice. Infect Immun 2000; 68:2925–9.
Llorente L, De La Fuente H, Richaud-Patin Y, et al. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology. Immunol Lett 2000; 74:239–44.
Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 2000; 49:1451–8.
Ilyas R, Wallis R, Soilleux EJ, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology 2011; 216:126–31.
Wilke T, Boettger B, Berg B, et al. Epidemiology of urinary tract infections in type 2 diabetes mellitus patients: An analysis based on a large sample of 456,586 German T2DM patients. J Diabetes Complications 2015; 29:1015–23.
Mor A, Dekkers OM, Nielsen JS, Beck-Nielsen H, Sørensen HT, Thomsen RW. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. Am J Epidemiol 2017; 186:227–36.
Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 2008; 31:1541–5.
Thomsen RW, Riis AH, Kjeldsen S, Schønheyder HC. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. J Infect 2011; 63:8–16.
Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications 2012; 26:501–5.
Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J Diabetes Complications 2012; 26:513–6.
Mor A, Berencsi K, Nielsen JS, et al. Rates of community-based antibiotic prescriptions and hospital-treated infections in individuals with and without type 2 diabetes: a Danish nationwide cohort study, 2004–2012. Clin Infect Dis 2016; 63:501–11.
Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018; 41:2127–35.
Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol 2016; 4:148–58.
Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care 1995; 18:1415–27.
Bartelink ML, Hoek L, Freriks JP, Rutten GE. Infections in patients with type 2 diabetes in general practice. Diabetes Res Clin Pract 1998; 40:15–9.
Li W, Katzmarzyk PT, Horswell R, et al. HbA1c and all-cause mortality risk among patients with type 2 diabetes. Int J Cardiol 2016; 202:490–6.
Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, . Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open 2017; 7:e015949.
Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med 2003; 163:1825–9.
Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009; 32:1153–7.
Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care 2011; 15:R173.
Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367:1108–18.
Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health 2015; 73:43.
Berk R, MacDonald JM. Overdispersion and Poisson regression. J Quant Criminol 2008; 24:269–84.
Payne EH, Hardin JW, Egede LE, Ramakrishnan V, Selassie A, Gebregziabher M. Approaches for dealing with various sources of overdispersion in modeling count data: Scale adjustment versus modeling. Stat Methods Med Res 2017; 26:1802–23.