Research Article - (2024) Volume 19, Issue 1
INFLUENCE OF MANUAL DIAPHRAGM RELEASE COMBINED WITH CONVENTIONAL BREATHING EXERCISES AND PRONE POSITIONING ON PULMONARY FUNCTIONS IN WOMEN WITH COVID-19
Mona Abdelraouf Ghallab1*, Ahmed Fekry Salman2, Neveen Mohammed Nawar3, Karim Ibrahim Saafan1, Amira Ismail Mostafa, Kerolous Ishak Shehata Kelini5,6, ., Shaimaa Mohamed Mabrouk Bondok7,8, . and Mona Ahmed Abdelwahab1*Correspondence: Mona Abdelraouf Ghallab, Department of Physical Therapy for, Cardiovascular/Respiratory Disorders & Geriatrics, Faculty of Physical Therapy, Cairo University, Egypt, Email:
2Assistant Professor, Al-Ahliyya Amman University, Physiotherapy department, Faculty of Allied Medical Sciences, Jordan
3Department of Physical Therapy for intensive care unit at Damanhur National Medical Institute, Egypt
4Department of chest at Kasr El Aini Teaching Hospital, Faculty of Medicine, Cairo University, Egypt
5Department of Physical Therapy, Faculty of Applied Medical Sciences, Al-Zaytoonah University of Jordan, Amman, Jordan
6Department of Physical Therapy for Womens Health, Faculty of Physical Therapy, October 6 University, Egypt
7Department of Orthotics & Prosthetics, Delta Technological University, Queisna, Egypt
8Department of Physical Therapy, Benha Specialized Children Hospital, Egypt
Received: 15-Feb-2024 Published: 20-Feb-2024
Abstract
Background: Manual noninvasive respiratory techniques gained interest to treat respiratory pathologies related to COVID 19.
Purpose: This study designed to determine the combined impact of manual diaphragmatic release technique with the impact of traditional breathing exercises as well as prone positioning on pulmonary function outcomes such as (FVC, FEV1, PEF, FEV1/FVC, FEF25, FEF50, FEF75 in addition to FEF25/75).
Methods: Forty females were randomized into two groups. Group A was given manual diaphragm release with conventional breathing exercises and prone positioning. Group B was given traditional breathing exercises as well as prone positioning. Both groups took their prescribed medications. They were aged from 35 to 45 years and with moderate COVID-19 illness. Any cases with mild and severe COVID-19 illness, ICU admission, and chest diseases were excluded.
Results: Compared to pre-treatment, both groups demonstrated statistically significant enhancement across all outcome measures. Both groups illustrate improving these measures with favor to group A. After the treatment, group A had significantly higher FVC, FEV1, and FEV1/FVC values compared with group B (p < 0.05). There was a significant improvement in values of PEF, PEF 25%, PEF 50%, PEF 75% as well as PEF 25-75% of group A compared to that of group B after treatment (p < 0.05).
Conclusion: It could be concluded that adding diaphragmatic release to conventional breathing exercise can improve pulmonary function parameters which is closely related to improve chest expansion, dyspnea and diaphragm excursion, thus in turn improve overall patient quality of life.
Keywords
Breathing Exercise COVID-19. Manual diaphragmatic reléase. Pulmonary function
Introduction
In December of 2019, a new coronavirus emerged on Earth; on the eleventh of March 2020, the World Health Organization named the global outbreak of COVID-19 a pandemic requiring a public health emergency (Eggmann et al., 2021). COVID 19 leads to various symptoms including fever, dyspnea, cough, excessive secretions on the airway. Patients also suffer from weakening in body functions as well as structures, activity limitations, decreased pulmonary function, and decline of strength of the respiratory and extremity muscles which in turn affect their capability to accomplish activities of daily living. Because of the virus's primary impact on the respiratory system, physical therapy in addition to rehabilitation programs are essential for managing COVID-19 symptoms. Reducing dyspnea symptoms, increasing lung capacity, preventing consequences from respiratory failure and immobility, and facilitating functional recovery are all goals associated with early respiratory physiotherapy (Zhu et al., 2020).
The goal of pulmonary rehabilitation is to alleviate the physical and mental symptoms of lung disorders, increase oxygen exchange, avoid lung collapse, and decrease the requirement for artificial ventilation through the use of breathing exercises along with respiratory muscle training techniques like diaphragmatic breathing, pursed-lip breathing, relaxation techniques, in addition to body positioning exercises. Respiratory rehabilitation is a crucial part of COVID-19 survivors’ treatment combined with early respiratory rehabilitation has the potential to decrease hospital stays while improving overall lung function (Kader et al., 2022).
Patients with respiratory diseases may see an improvement in lung function, diaphragm as well as rib cage mobility, and even immunological function after receiving certain manual procedures. To facilitate stronger and more efficient contractions, a manual technique called the diaphragmatic release technique is used. This technique aims to indirectly lengthen the fibers of the diaphragmatic muscle. Improving thoracic mobility and lung function have both been achieved with the (DR) technique (Elnaggar et al., 2019).
An increasingly common strategy for improving oxygenation and preventing ventilator-related lung injuries is to prone awake COVID-19 patients. This is since while in a prone position, the central anterior regions of the patient's chest are compressed, that increases cardiac output and improves pulmonary respiration (Rahmani et al., 2020).
The angiotensin-converting enzyme 2 (ACE2) is an entry receptor for the SARS-CoV-2 virus within skeletal muscles and lung alveolar cells. Among the several skeletal muscles that affect lung capacity and respiratory performance, the diaphragm stands out as the primary muscle responsible for breathing (Nagy et al., 2022). Even though COVID-19 has an important impact on the diaphragm muscle as well as the manual DR technique has its advantages, it is unclear what impact this technique may have in enhancing pulmonary function measures like FVC, FEV1, PEF, FEV1/FVC, PEF25, PEF50, PEF75, as well as PEF25/75 in COVID-19 patients.
There is limited evidence concerning the combined impact of diaphragmatic release with conventional breathing exercise and prone position on pulmonary function parameters of COVID-19 patients. It was hypothesized that manual DR in combination with traditional breathing exercises as well as prone positioning would help to restore pulmonary function parameters in patients with post-COVID-19. So, this study was designed to examine the combined impact of manual diaphragmatic release technique with traditional breathing exercises as well as prone positioning compared with traditional breathing exercises as well as prone positioning alone on pulmonary function parameters in COVID-19 women.
A recent study held to examine whether or not manual diaphragm release combined with traditional breathing exercises as well as prone positioning is more effective than traditional breathing exercise and prone position alone in preserving lung functions in women with COVID-19.
Methods
Study design and setting
This 3-week, retrospective single-blinded randomized controlled trial (RCT) evaluated the combined impact of manual DR with breathing exercise as well as prone position in women with COVID-19 on pulmonary function parameters (FVC, FEV1, FEV1/FVC, PEF, PEF25, PEF50, PEF75, PEF25/75). The study was conducted in July 2022 and ended in September 2022. Participants were diagnosed by a clinician while hospitalized at Damanhour National Medical Institute. Prior to participating in the study, patients gave their informed consent.
Participants were randomized 1:1 allocation ratio by a researcher not involved in the application of the interventions into a study group (n = 20) and a control group (n = 20) following description of the study details. simple randomization was used. A blinded research assistant numbered the opaque sealed envelopes in sequential order and distributed them to the patients. Assigning the groups was known to the study team, but the participants as well as the researcher who did the physiotherapy were unaware of it.
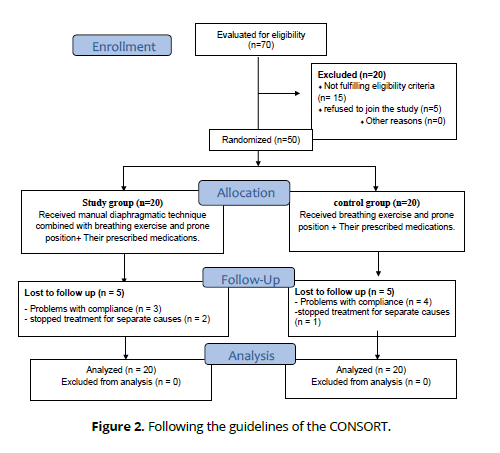
The study group was given manual DR with breathing exercise and prone positioning along with their drugs, whereas the control group was given breathing exercise and prone positioning along with their drugs (Fig. 1). All procedures adhered to the principles outlined in the Declaration of Helsinki and the Consolidated Standards of Reporting Trials (CONSORT). The research was given approval by the Faculty of Physical Therapy's ethics committee board (number (P.T.REC/012/004575) and registered with ClinicalTrials.gov (NCT04919031).
Sample size calculation
Utilizing the G*POWER statistical program (version 3.1.9.2; Universitat Kiel, Germany), we were able to determine that a total of 20 subjects per group was necessary for the study. We used an allocation ratio of N2/N1 = 1, α=0.05, with power = 80% and effect size was 0.91. (Figure 1).
Eligibility criteria
The study involved patients who met the subsequent criteria:
• Women with moderate COVID-19 illness diagnosed by a physician.
• Patients with o2 saturation >94%Nonsmoker subjects.
• Between the ages from 35 to 45 years.
• Body mass index (25 – 34) kg/m2.
The following flow diagram shows the research participants' path through the study: allocation, intervention, as well as follow-up. Forty individuals were able to complete the study. (Figure 2).
Patients were excluded if they had:
• Unstable hemodynamic status.
• Acute respiratory failure requiring intubation and impaired consciousness.
• Inability to collaborate with prone positioning with refusal.
• Change of mental status hindering response to instructions.
• Poorly controlled hypertension (Mean systolic BP > 140 mmhg and \ or diastolic BP > 40 mmhg).
• Patients who take continuous o2 supplementation.
• Smoking.
• Other chest diseases as (COPD-asthma-tuberculosis-cancer).
• Male patients.
Evaluation
Clinical examination as well as medical history taking
The Physician diagnoses the case according to the clinical examination and medical history and excludes patients not suitable for the study.
Outcomes Measured
The following Parameters was measured using the Spirometry:
• Forced expiratory volume in first second (FEV1).
• Forced vital capacity (FVC), when exhaling rapidly, what is the greatest volume of air that can be expired.
• FEV1/FVC ratio
• Peak expiratory flow (PEF), it is the maximum amount of air that can be expired when you blow out your nose as quickly as possible.
• Forced expiratory flow, sometimes referred to as mid-expiratory flow; the rates are provided at 25%, 50%, as well as 75% FVC.
Infection control
Washing hands carefully between patients is a must. The patient is responsible for disposing of the bacterial-viral one-use filters after testing is complete. If the test were held to an infectious patient, It would be done after the session, and the instruments would need to be cleaned, sanitized or have parts changed before it could be utilized again. (Moore., 2012).
Before a Spirometry test
• Educate the patient how to perform the test and supervised trial should be done to ensure patient understanding for the test steps.
• The patient should be instructed not to take any breathing medicines before the test.
• The therapist should be aware that this test recommend effort and expect that the patient may become tired.
• If she becomes light-headed or dizzy during this test, immediately stop the test.
• The patient should avoid eating large meals within 2h before the test, strenuous exercise for at least 30 min before the test, drinking coffee, tea, chocolate, or further items involving caffeine on the day of the test.
• The patient should avoid wearing any jewelry that might affect her breathing.
• Make sure her mouth can snugly fit around the test mouthpiece by not wearing dentures or any other removable dental items.
• Avoid wearing tight clothes that may affect her deep breathing. (Moore., 2012).
The assessments of pulmonary function test by spirometry according to Graham, (2019)
➢ The test was held by using a portable spirometer Spriox plus.
➢ Each patient data (age- weight-height-sex) was recorded into the apparatus database before beginning the test.
➢ The patient sat with nearly vertical thorax and the chin slightly elevated.
➢ The patient was instructed about how to perform the test.
➢ A private mouthpiece was given for each patient.
➢ For a few seconds, the patient was instructed to exhale forcefully into the mouthpiece after taking a deep breath. It's important that her lips create a seal around the mouthpiece to avoid air leaks out.
➢ At the end of exhalation, she removed the mouthpiece and breathes normally.
Interventions
Pharmacological treatment
A pulmonologist gave each patient in both groups the following medication protocol:
Group A intervention
1-Manual diaphragmatic release technique
Group (A): along with their prescribed medications, they received manual diaphragmatic release technique (3 times per week for 3 weeks).
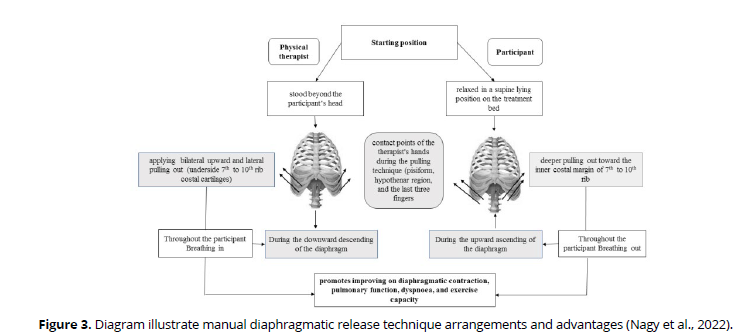
The patient was asked to lie in a supine position on the plinth. The therapist sat behind the patient's head with manual contact of patient's costal cartilages from seventh to tenth ribs using the pisiform, ulnar, and pads of the last three fingers of both hands. In inspiratory phase, the therapist applies gentle upward and lateral pulling up by her hands to elevate the ribs. As the patient exhaled, the therapist maintained resistance as she pushed further into the inner costal edge. The therapist's contact depth inside the chest was progressively deepened in subsequent breathing cycles. (Figure 3).
Progression of sessions
In the first week, this technique was applied for two sets each set five deep breaths with a one-minute of rest between them, then in the second and third weeks in 2 sets each 10 deep breaths with a five-minute of rest among them.
2- Conventional breathing exercises
• Diaphragmatic breathing in combination with pursed lips breathing exercises.
The Patient was requested to lie in a supine position and the therapist placed one hand under the anterior costal cartilage to induce inspiratory diaphragmatic breathing. The patient breathed in slowly as well as deeply through the nose while maintaining the shoulders and upper chest in relaxed state. Then purse the lips and made the expiration with counting four in her mind. Two sets were done while seated and two sets while lying supine; each set included two deep diaphragmatic breaths, and there was a two-minute of rest among each set (Seo., 2017).
• Breathing exercises combined with upper extremity motion
Two sets were performed while seated and two sets while standing for these exercises. Two sets of deep breathing followed by upper extremity motion activity constituted each set. Each exercise lasted one minute, with a twominutes of rest between each one, for a total of fifteen minutes. The exercises lasted a total of fifteen minutes, with each set lasting one minute and separated by two-minute rests. Throughout the upper-extremity exercises, patients were asked to inhale deeply from the nose with abduction (from 0 to 90 degree) or flexion (from 0 to 180 degree) and exhale through the mouth to return to the starting position (Costa et al., 2011).
3-Prone positioning
The patient was instructed to lie down in a prone position for a minimum of two hours and then to alternate between different positions, each of which should be sustained for 30 to 120 minutes: upright sitting, left lateral decubitus, as well as prone (Downing et al., 2021).
Group B intervention
Group B was given traditional breathing exercises (3 times a week), prone positioning (day-to-day) as well as pharmacological treatment.
Statistical analysis
To compare the participant's characteristics between the groups, an independent-test was used. We used the Shapiro-Wilk test to make sure the data was normally distributed. The homogeneity of variances among groups was tested using Levene's test. To examine the impact of treatment on lung function, a mixed MANOVA was performed. Following that, multiple comparisons were performed utilizing post hoc tests with the Bonferroni correction. All statistical tests were set to have a significance threshold of p < 0.05. The data was analyzed using SPSS version 25 for Windows, which is a statistical tool developed by IBM SPSS in Chicago, IL, USA.
Results
Patients characteristics
The group A and B subjects' characteristics are shown in (Table 1). Age, height, weight, BMI, and recurrence did not differ significantly (p > 0.05) among groups.
| Group A | Group B | MD | t- value | p-value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Age (years) | 39.30 ± 2.93 | 38.85 ± 2.90 | 0.45 | 0.48 | 0.62 |
| Weight (kg) | 75.40 ± 5.17 | 76.25 ± 4.77 | -0.85 | -0.51 | 0. 59 |
| Height (cm) | 159.90 ± 3.56 | 160.40 ± 2.62 | -0.5 | -0.54 | 0.61 |
| BMI (kg/m²) | 29.48 ± 1.59 | 29.63 ± 1.58 | -0.15 | -0. 30 | 0.76 |
| Recurrence | 3.80 ± 1.11 | 3.55 ± 0.83 | 0.25 | 0.81 | 0.42 |
SD, Standard deviation; MD, Mean difference; p value, Probability value.
Impact of treatment on pulmonary function:
Treatment and time interacted significantly (F = 5.86, p = 0.001), according to mixed MANOVA. The treatment had a statistically significant main impact (F = 6.39, p = 0.001). The main effect of time was statistically significant (F = 53.59, p = 0.001).
Within group comparison
After treatment, both groups' FVC, FEV1, and FEV1/FVC values were significantly higher than their baseline levels (p > 0.001). In group A, the percentage changes for FVC, FEV1, and FEV1/FVC were 166, 209.8, and 18.5%, correspondingly, while in group B, the corresponding percentage changes were 100, 117.5, and 9.5%. In Table 2.
| Before treatment | After treatment | MD | % of change | p value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| FVC (L) | |||||
| Group A | 0.53 ± 0.18 | 1.41 ± 0.42 | -0.88 | 166 | 0.001 |
| Group B | 0.51 ± 0.22 | 1.02 ± 0.60 | -0.51 | 100 | 0.001 |
| MD | 0.02 | 0.39 | |||
| p value | p = 0.84 | p = 0.02 | |||
| FEV1 (L) | |||||
| Group A | 0.41 ± 0.14 | 1.27 ± 0.37 | -0.86 | 209.8 | 0.001 |
| Group B | 0.40 ± 0.17 | 0.87 ± 0.51 | -0.47 | 117.5 | 0.001 |
| MD | 0.01 | 0.4 | |||
| p value | p = 0.98 | p = 0.007 | |||
| FEV1/FVC (%) | |||||
| Group A | 76.72 ± 4.42 | 90.91 ± 5.84 | -14.19 | 18.5 | 0.001 |
| Group B | 78.77 ± 6.78 | 86.27 ± 5.86 | -7.5 | 9.5 | 0.001 |
| MD | -2.05 | 4.64 | |||
| p value | p = 0.26 | p = 0.01 | |||
There was a significant increase in PEF, PEF 25%, PEF 50%, PEF 75% and PEF 25- 75% after treatment when compared to that before treatment in both groups (p > 0.01). The percentage of change of PEF, PEF 25%, PEF 50%, PEF 75% and PEF 25-75% of group A was 203.6, 256.5, 305.8, 128.9 and 70.8%respectively and that in group B was 114.1, 131.8, 200, 85.7 and 25.9% respectively (Table 3).
| Before treatment | After treatment | MD | % of change | p value | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| PEF (L/s) | |||||
| Group A | 0.83 ± 0.32 | 2.52 ± 0.79 | -1.69 | 203.6 | 0.001 |
| Group B | 0.85 ± 0.36 | 1.82 ± 0.91 | -0.97 | 114.1 | 0.001 |
| MD | -0.02 | 0.7 | |||
| p value | p = 0.88 | p = 0.01 | |||
| PEF 25% (L/s) | |||||
| Group A | 0.69 ± 0.39 | 2.46 ± 0.46 | -1.77 | 256.5 | 0.001 |
| Group B | 0.66 ± 0.28 | 1.53 ± 0.33 | -0.87 | 131.8 | 0.001 |
| MD | 0.03 | 0.93 | |||
| p value | p = 0.82 | p = 0.001 | |||
| PEF 50% (L/s) | |||||
| Group A | 0.52 ± 0.19 | 2.11 ± 0.85 | -1.59 | 305.8 | 0.001 |
| Group B | 0.49 ± 0.17 | 1.47 ± 0.73 | -0.98 | 200 | 0.001 |
| MD | 0.03 | 0.64 | |||
| p value | p = 0.71 | p = 0.01 | |||
| PEF 75% (L/s) | |||||
| Group A | 0.45 ± 0.23 | 1.03 ± 0.39 | -0.58 | 128.9 | 0.001 |
| Group B | 0.42 ± 0.22 | 0.78 ± 0.27 | -0.36 | 85.7 | 0.001 |
| MD | 0.03 | 0.25 | |||
| p value | p = 0.67 | p = 0.02 | |||
| PEF 25-75% (L/s) | |||||
| Group A | 1.54 ± 0.43 | 2.63 ± 0.92 | -1.09 | 70.8 | 0.001 |
| Group B | 1.66 ± 0.37 | 2.09 ± 0.50 | -0.43 | 25.9 | 0.009 |
| MD | -0.12 | 0.54 | |||
| p value | p = 0.37 | p = 0.02 | |||
Between group comparison
After the treatment, group A had significantly higher FVC, FEV1, and FEV1/ FVC values compared to group B (p < 0.05). After the treatment, group A had significantly higher PEF values (p < 0.05) compared to group B, with increases of 25%, 50%, 75%, and 25-75%. (Tables 2-3).
Discussion
This is the first study aimed to examine the impact of adding manual diaphragmatic release technique to traditional breathing exercise as well as prone position on lung function parameters (FVC, FEV1, FEV1/FVC, PEF, PEF25, PEF50, PEF75, PEF25/75). In accordance with our hypothesis, this study has revealed that manual diaphragm release when added to traditional breathing exercises has induced significant improvements in pulmonary function parameters assessed by a portable spirometer.
Patients with COVID-19 commonly suffer from shortness of breath, fatigue, and respiratory muscle weakness (Mizrahi et al., 2020). However, we granted the prominence impact of traditional breathing exercise as well as prone position on COVID- 19 respiratory symptoms but according to several studies, it was found that that manual diaphragmatic release accompanied with traditional breathing exercise offered extra clinical advantage by performing a statistically significant enhancement in pulmonary function parameters compared with breathing exercise alone. We also found improvement of oxygen saturation through pulse oximeter attached to the patient finger, better chest expansion and reduced dyspnea reported by patients. Patients also reported better quality of life as they can exert effort without respiratory complaints.
According to Yilmaz Yelvar et al. (2016), lung function as well as inspiratory muscle strength were both improved in patients with severe chronic obstructive pulmonary disease after just one session of manual therapy that involved diaphragmatic release. Our findings are in line with theirs. Parasympathetic nervous system activity followed diaphragmatic release. As a result of its relaxation-regulating functions, the autonomic nervous system improves lung function as well as oxygen saturation while decreasing fatigue, dyspnea, and breathing rate.
The study conducted by Nair et al. in 2019 demonstrated that diaphragmatic release, which activates the parasympathetic system, resulted in enhanced diaphragm excursion, oxygen saturation, and reduced bronchospasm, respiratory rate, work of breathing, as well as dyspnea. Relaxation, increased thoracic mobility, and improved chest wall compliance are all outcomes of manual treatment. Decreased breathing effort and improved muscular function are the results of a relaxed and flexible thoracic cage.
FVC is when exhaling rapidly, what is the greatest volume of air that can be expired. FVC is utilized to assess lung function, progression of lung disease and evaluate the effectiveness of treatment (Rivero-Yeverino 2019). PEF25–75% is a sensitive parameter for evaluating the function of peripheral airway function (Szefler et al., 2020), but it may be reduced due to decreased lung volume rather than from airways disease (Quanjer et al., 2014). Manual diaphragmatic release in addition to conventional breathing exercise can significantly increase FVC, FEV1, PEF, FEF25/75. This is consistent with the findings of González- According to Álvarez et al. (2015), who discovered that diaphragm stretching improved pulmonary function, spirometric measurements of FVC and FEV1, and maximal respiratory pressures, as well as there were significant changes in FVC and FEV1 5 and 20 minutes following the stretching technique. In addition, diaphragm stretching improved maximal respiratory pressures. We reported an improvement in O2 saturation and decline in breathlessness and fatigue in the diaphragm release group, these can be justified by the fact that, after three weeks of manual diaphragm release, there is a large enhancement in the respiratory muscles’ strength as well as the lung function.
Rocha et al. (2015) demonstrated individuals with stable COPD had improvements in diaphragm mobility, exercise ability, and inspiratory capacity after using the Manual Diaphragm Release Technique, adding strength to our findings.
Consistent with our findings, Abdelaal et al. (2015) also observed that the Diaphragmatic Release technique, when applied, considerably improved FVC, FEV1, and 6MWT. This study's findings are in line with those of Marizeiro et al. (2018), who showed that diaphragmatic release techniques increase the range of motion (ROM) of the lumbar spine, flexibility of the posterior chain muscles, and mobility of the chest wall in inactive young women. Strength of the respiratory muscles and lumbar flexion showed no change.
Additionally, compared to traditional breathing and prone positioning isolated, manual diaphragm release resulted in a substantially greater improvement in lung vital capacity in COVID-19 women. There was a positive correlation between vital capacity, FEV1, FVC, as well as FEV1/FVC, which led to an improvement in upper chest expansion as well. Chest expansion is a reliable measure of lung function as well as chest mobility (Reddy et al., 2019). This strengthens the hypothesis that after diaphragm release there would be more improvement in ventilatory function and possibly led to decrease breathing work. Additionally, previous studies demonstrated that diaphragm release improved chest expansion.
The results of this research supported those of Leonés-Macías E et al. (2018), who found that individuals with asthma experienced an increase in rib cage mobility and flexibility after receiving a diaphragm stretching procedure.
In agreement with Courtney et al., (2019) Showed that manual therapy combined with breathing retraining in asthma patients with dysfunctional breathing can reduce symptoms and complications. This study's results corroborated those of Elnaggar et al. (2019), who found that traditional respiratory retraining, the thoracic lymphatic pump approach, or diaphragmatic release technique during twelve treatment sessions successfully decreased asthma symptoms and improved FVC, FEV1, and diaphragmatic movement in children. He proposed diaphragmatic release technique as an alternative that could be more beneficial.
Gentle manual compression over the insertion of anterior diaphragmatic fiber (the underpart of the 7th to the 10th costal cartilages) led to stretching the diaphragm fiber at this zone. Thus increase the active force of diaphragm during contraction according to force-length relationship which means by increasing the length of muscle, the active force increases until reach the maximum like limb muscles. The previous explanation illustrates the mechanism of action of diaphragmatic release from the biomechanical point of view suggesting that it could improve the mechanical efficiency of diaphragm contraction resulting in patients reported benefits which reflect in their life (Ahmad et al., 2023).
Through its biomechanical effects on distant tissues like the cervical as well as lumbar spine, diaphragmatic stretching not only improves respiratory function but also enhances postural function (Nair et al., 2019).
Since there has been little research on diaphragmatic release in patients having moderate COVID-19 symptoms, we were motivated to pay attention to its superiority when combined with the standard breathing exercise program for alleviating symptoms and enhancing patients' quality of life. It is believed that diaphragmatic release is an indirect means of improving the efficiency of muscle contractions since it enhances respiratory function, decreases sympathetic excitability, and thus decreases dyspnea (González-Álvarez et al., 2016).
Study limitations
Since the majority of the COVID-19 patients at the hospital where the study was carried out were female, the selection of participants was restricted to females with moderate COVID-19. This may have restricted the scope of the results. It is unethical that patients will not be able to obtain their physiotherapy because there is no actual control group that just receives medical treatment. But there are many positive aspects to this study as well. Manual diaphragm release, in addition to traditional breathing exercises, has never been the subject of a randomized trial until now. Furthermore, it is important to evaluate these advantages in comparison to those that can be achieved by the more traditional methods of deep breathing and prone positioning. An easily taught manual approach that patients accept and can generate extremely good results was employed in the present study.
Conclusion
This study offers evidence on the impact of diaphragmatic release technique with moderate COVID-19 patients. The findings revealed that adding diaphragmatic release to conventional breathing exercise can improve pulmonary function parameters which is closely related to improve chest expansion, dyspnea and diaphragm excursion, thus in turn improve overall patient quality of life. Our findings may help the researchers who are interested in the manual therapy role in the treatment plan of COVID-19.
References
Abdelaal A A., Ali M M., and Hegazy I. M. (2015): Effect of diaphragmatic and costal manipulation on pulmonary function and functional capacity in chronic obstructive pulmonary disease patients. Int J Med Res Health Sci.,4(4): 841-847.
Ahmad AM, Nawar NM, Dabess HM, Gallab MA. Effect of diaphragm manual release versus conventional breathing exercises and prone positioning on physical functional performance in women with COVID-19. A randomized trial. J Bodyw Mov Ther. 2023 Apr 21. doi: 10.1016/j.jbmt.2023.04.064.
Costa D, Cancelliero KM, Ike D, Laranjeira TL, Pantoni CB, Borghi-Silva A. Strategy for respiratory exercise pattern associated with upper limb movements in COPD patients. Clinics (Sao Paulo). 2011;66(2):299-305. doi: 10.1590/s1807-59322011000200020.
Downing J, Cardona S, Alfalasi R, Shadman S, Dhahri A, Paudel R, Buchongo P, Schwartz B, Tran QK. Predictors of intubation in COVID-19 patients undergoing awake proning in the emergency department. Am J Emerg Med. 2021 Nov; 49:276-286. doi: 10.1016/j.ajem.2021.06.010.
Eggmann S, Kindler A, Perren A, Ott N, Johannes F, Vollenweider R, Balma T, Bennett C, Silva IN, Jakob SM. Early Physical Therapist Interventions for Patients With COVID-19 in the Acute Care Hospital: A Case Report Series. Phys Ther. 2021 Jan 4;101(1):pzaa194. doi: 10.1093/ptj/pzaa194.
Elnaggar RK, Shendy MA, Mahmoud MZ. Prospective Effects of Manual Diaphragmatic Release and Thoracic Lymphatic Pumping in Childhood Asthma. Respir Care. 2019 Nov;64(11):1422-1432. doi: 10.4187/respcare.06716.
Elnaggar RK, Shendy MA, Mahmoud MZ. Prospective Effects of Manual Diaphragmatic Release and Thoracic Lymphatic Pumping in Childhood Asthma. Respir Care. 2019 Nov;64(11):1422-1432. doi: 10.4187/respcare.06716.
González-álvarez FJ, Valenza MC, Cabrera-Martos I, et al.Effects of a diaphragm stretching technique on pulmonary function in healthy participants: a randomized-controlled trial.Int J Osteopath Med. 2015;18(1):5–12.
González-Álvarez FJ, Valenza MC, Torres-Sánchez I, Cabrera-Martos I, Rodríguez-Torres J, Castellote-Caballero Y. Effects of diaphragm stretching on posterior chain muscle kinematics and rib cage and abdominal excursion: a randomized controlled trial. Braz J Phys Ther. 2016 Jun 16;20(5):405-411. doi: 10.1590/bjpt-rbf.2014.0169.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE, Rosenfeld M, Stanojevic S, Swanney MP, Thompson BR. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019 Oct 15; 200(8):e70-e88. doi: 10.1164/rccm.201908-1590ST.
Kader M, Hossain MA, Reddy V, Perera NKP, Rashid M. Effects of short-term breathing exercises on respiratory recovery in patients with COVID-19: a quasi-experimental study. BMC Sports Sci Med Rehabil. 2022 Apr 5;14(1):60. doi: 10.1186/s13102-022-00451-z.
Leonés-Macías E., Torres-Sánchez I., Cabrera-Martos I., Ortiz-Rubio A., López- López L., and Valenza M C., (2018): Effects of manual therapy on the diaphragm in asthmatic patients. A randomized pilot study. International Journal of Osteopathic Medicine, 29, 26-31.
Marizeiro DF, Florêncio ACL, Nunes ACL, Campos NG, Lima POP. Immediate effects of diaphragmatic myofascial release on the physical and functional outcomes in sedentary women: A randomized placebo-controlled trial. J Bodyw Mov Ther. 2018 Oct;22(4):924-929. doi: 10.1016/j.jbmt.2017.10.008.
Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, Keshet A, Shamir-Stein N, Shalev V, Zohar AE, Chodick G, Segal E. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 2020 Dec 4;11(1):6208. doi: 10.1038/s41467-020-20053-y.
Moore V C., (2012):Spirometry step by step. Breathe 2012, 8(3), 232-240. doi: 10.1183/20734735.5217-2011.
Nagy EN, Elimy DA, Ali AY, Ezzelregal HG, Elsayed MM. Influence of Manual Diaphragm Release Technique Combined with Inspiratory Muscle Training on Selected Persistent Symptoms in Men with Post-Covid-19 Syndrome: A Randomized Controlled Trial. J Rehabil Med. 2022 Oct 20;54:jrm00330. doi: 10.2340/jrm.v54.3972.
Nair A, Alaparthi GK, Krishnan S, Rai S, Anand R, Acharya V, Acharya P. Comparison of Diaphragmatic Stretch Technique and Manual Diaphragm Release Technique on Diaphragmatic Excursion in Chronic Obstructive Pulmonary Disease: A Randomized Crossover Trial. Pulm Med. 2019 Jan 3;2019:6364376. doi: 10.1155/2019/6364376.
Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J. 2014 Apr;43(4):1051-8. doi: 10.1183/09031936.00128113.
Rahmani F, Salmasi S, Rezaeifar P. Prone Position Effects in the Treatment of Covid-19 Patients. Caspian J Intern Med. 2020 Fall;11(Suppl 1):580-582. doi: 10.22088/cjim.11.0.580.
Reddy RS, Alahmari KA, Silvian PS, Ahmad IA, Kakarparthi VN, Rengaramanujam K. Reliability of Chest Wall Mobility and Its Correlation with Lung Functions in Healthy Nonsmokers, Healthy Smokers, and Patients with COPD. Can Respir J. 2019 Feb 25;2019:5175949. doi: 10.1155/2019/5175949.
Rivero-Yeverino D. Espirometría: conceptos básicos [Spirometry: basic concepts]. Rev Alerg Mex. 2019 Jan-Mar;66(1):76-84. Spanish. doi: 10.29262/ram.v66i1.536.
Rocha T, Souza H, Brandão DC, Rattes C, Ribeiro L, Campos SL, Aliverti A, de Andrade AD. The Manual Diaphragm Release Technique improves diaphragmatic mobility, inspiratory capacity and exercise capacity in people with chronic obstructive pulmonary disease: a randomised trial. J Physiother. 2015 Oct;61(4):182-9. doi: 10.1016/j.jphys.2015.08.009.
Seo K, Hwan PS, Park K. The effects of inspiratory diaphragm breathing exercise and expiratory pursed-lip breathing exercise on chronic stroke patients' respiratory muscle activation. J Phys Ther Sci. 2017 Mar;29(3):465-469. doi: 10.1589/jpts.29.465.
Szefler SJ, Goldstein S, Vogelberg C, Bensch GW, Given J, Jugovic B, Engel M, Moroni-Zentgraf PM, Sigmund R, Hamelmann EH. Forced Expiratory Flow (FEF25-75%) as a Clinical Endpoint in Children and Adolescents with Symptomatic Asthma Receiving Tiotropium: A Post Hoc Analysis. Pulm Ther. 2020 Dec;6(2):151-158. doi: 10.1007/s41030-020-00117-6.
Yilmaz Yelvar GD, Çirak Y, Demir YP, Dalkilinç M, Bozkurt B. Immediate effect of manual therapy on respiratory functions and inspiratory muscle strength in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016 Jun 20;11:1353-7. doi: 10.2147/COPD.S107408.
Zhu Y, Wang Z, Zhou Y, Onoda K, Maruyama H, Hu C, Liu Z. Summary of respiratory rehabilitation and physical therapy guidelines for patients with COVID-19 based on recommendations of World Confederation for Physical Therapy and National Association of Physical Therapy. J Phys Ther Sci. 2020;32(8):545-549. doi: 10.1589/jpts.32.545.