Research - (2024) Volume 19, Issue 5
Longterm Health Outcomes And Quality Of Life In Women With Gestational Diabetes A Systematic Review
Rawan Deham Aledeilah1*, Norah Ayed Ayash Alenezi2, Abdulaziz Saud Alhelal3, Nouf Ayed A Alenezi4, Basmah Darzi Abdullah Alanezi5, Maryam Fahad Alenezi6, Eman Roshdy Mohamed7, Kholoud Obeid H Al Bathaly8, Omar Mohammed F. Aldosari9, Nasserullah Kulib Albalawi10, Abdulrahman Ahmed M Alnujaydi11 and Omaima Anan Omer Hamid12*Correspondence: Rawan Deham Aledeilah, Senior Registrar of Family Medicine, Aziziayah Primary health care center, Arar, Saudi Arabia, Email:
2Family Medicine Resident, Northern Borders Health Cluster, Arar, Saudi Arabia
3Bachelor degree in Medicine and Surgery, College of Medicine, King Saud bin Abdulaziz University for health sciences, Riyadh, Saudi Arabia
4General Physician, Maternity and children Hospital, Arar, Saudi Arabia
5Specialist Nurse, Prince Abdulaziz Bin Musaad Hospital, Arar, Saudi Arabia
6Medical intern, Faculty of medicine, Almaarefa University, Saudi Arabia
7Assistant Professor of Public Health and community medicine, Sohag faculty of Medicine, Egypt
8General Practitioner, Janop Abo Mosa Primary Health Care center, Hafar al-Batin, Saudi Arabia
9General Practitioner, Family Medicine, Wadi Aldwassir General hospital, Riyadh, Saudi Arabia
10General Practitioner, Family Medicine, PHC center, Tabuk, Saudi Arabia
11Medical Intern, Faculty of Medicine, Northern Border University, Saudi Arabia
12MBBS, MD,MRCOG, Assistant professor, Department of Obstetrics and Gynaecology, Faculty of Medicine, Northern Border University, Saudi Arabia
Received: 01-Oct-2024 Published: 12-Oct-2024
Abstract
Objectives: To comprehensively assess the available evidence on the long-term health outcomes of women diagnosed with gestational diabetes (GDM).
Methods: A detailed computerized search of relevant databases was conducted to identify studies that met the inclusion criteria. The search encompassed PubMed, SCOPUS, Science Direct, Cochrane Library, and Web of Science to find pertinent research.
Results: Our analysis included nine studies with a total of 39,969 patients diagnosed with GDM. Women with a history of GDM are at a significantly increased risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) later in life. Factors such as early GDM diagnosis, obesity, and multifetal pregnancies further elevate this risk. Geographic and socioeconomic differences also influence the progression of T2DM. Additionally, GDM is associated with long-term cardiovascular complications, including impaired cardiac function and increased risk of myocardial infarction. Clinical management should include comprehensive postpartum monitoring and personalized interventions to mitigate these risks. Future research should focus on understanding the underlying mechanisms and developing targeted prevention strategies to improve long-term health outcomes in this high-risk population.
Conclusion: Women having a history of GDM are at a greatly elevated risk of acquiring T2DM and CVD later in life. These findings highlight the importance of providing proactive and customized postpartum care to reduce these risks.
Keywords
Gestational diabetes; Long-term health outcomes; Women's health; Maternal health; systematic review
Introduction
GDM is a complex condition characterized by glucose intolerance that is first recognized during pregnancy [1]. This condition affects a significant number of expectant mothers worldwide, with estimates suggesting that approximately 5-10% of pregnancies are complicated by GDM. While many women manage to return to normal glucose levels postpartum, emerging research indicates that the implications of GDM extend far beyond pregnancy, potentially influencing long-term health outcomes for women [2].
GDM typically resolves with the delivery of the baby; however, it is associated with a heightened risk of developing metabolic disorders later in life. Women diagnosed with GDM are significantly more likely to develop T2DM compared to those who do not experience this pregnancy complication. Studies have shown that the risk of developing T2DM can be as high as 50-70% within 5-10 years of a GDM diagnosis. This trajectory is concerning as T2DM is associated with various long-term complications, including cardiovascular disease, renal failure, and neuropathy [3].
The long-term repercussions of GDM extend to cardiovascular health. Women with a history of GDM have been shown to exhibit increased risks of hypertension and various forms of heart disease. Multiple studies have indicated that these women may have higher levels of inflammatory markers and altered lipid profiles, both of which are predispositions to cardiovascular complications. This creates a compelling need for regular cardiovascular screening and intervention strategies to mitigate these risks [4].
Another significant factor in the long-term health of women with a history of GDM is the increased risk of obesity. Postpartum weight retention is a common concern, with many women gaining more weight after pregnancy than they did during the nine months [5]. This weight gain can exacerbate the existing risk of developing T2DM and can also lead to an increase in metabolic síndrome-a cluster of conditions that raises the risk of heart disease and diabetes. Maintaining a healthy lifestyle, including regular physical activity and a balanced diet, becomes paramount for these women to manage their weight and reduce health risks [6].
The psychological dimension of coping with GDM should not be overlooked. Many women experience increased levels of anxiety and depression related to their GDM diagnosis. The pressures of managing blood glucose levels, diet, and lifestyle adjustments can take a toll on mental health during and after pregnancy. There is a growing recognition that mental health support is integral to the care of women with GDM, as psychological well-being is intricately linked to physical health outcomes [7].
Given the substantial long-term health risks associated with GDM, implementing preventive strategies is crucial. Healthcare providers play an essential role in educating women about their increased risk for T2DM and encouraging regular follow-up screenings postpartum. The American Diabetes Association recommends that women with a history of GDM undergo glucose testing 6-12 weeks after delivery, followed by testing every 1-3 years thereafter to ensure early detection and intervention [8].
In addition to medical monitoring, lifestyle interventions focusing on diet, exercise, and weight management are vital components of long-term health strategies for these women. Programs that promote sustainable changes in physical activity and nutrition can significantly mitigate the risks associated with previous GDM [9].
GDM is a common pregnancy complication that affects millions of women worldwide. While short-term outcomes like fetal macrosomia and hypoglycemia are well-established, the long-term health implications for women with GDM remain unclear. Previous studies have suggested an increased risk of developing T2DM, cardiovascular disease, and metabolic syndrome in women with a history of GDM. However, the extent and nature of these risks are still being investigated. Understanding the long-term health consequences of GDM is crucial for developing effective prevention and management strategies to improve the overall health and well-being of women who have experienced this condition.
Despite the prevalence of GDM and its potential impact on maternal health, there is a lack of comprehensive and up-to-date evidence regarding its long-term health outcomes. Existing studies may have limitations in terms of sample size, study design, and duration of follow-up, making it difficult to draw definitive conclusions. Therefore, there is a pressing need for a systematic review to synthesize the available literature and provide a more comprehensive understanding of the long-term health risks associated with GDM. The aim of this study is to conduct a systematic review of the existing literature on the long-term health outcomes of women with GDM. By synthesizing the available evidence, this review provided a comprehensive understanding of the risks associated with GDM and informed the development of effective prevention and management strategies.
Study Objectives
- To identify and assess the quality of studies investigating the long-term health outcomes of women with GDM.
- To extract relevant data from the included studies, including information on study design, sample characteristics, GDM diagnosis and management, and reported health outcomes.
- To synthesize the findings of the included studies and assess the overall quality of the evidence.
- To identify potential risk factors for long-term health complications in women with GDM.
- To discuss the implications of the findings for clinical practice, research, and public health.
Methods
This investigation follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards [10]. The goal is to thoroughly examine the long-term health consequences of GDM in women. An electronic literature search was undertaken using databases such as PubMed, Web of Science, SCOPUS, and Science Direct to locate relevant publications published in English that investigate the long-term health consequences associated with GDM. The search method included keywords related to GDM and associated health effects. Two independent reviewers reviewed the search results, chose qualifying papers, retrieved pertinent data, and evaluated the quality of the included research using acceptable evaluation procedures.
Eligibility Criteria
Inclusion and exclusion criteria were established to ensure the relevance and quality of the selected studies. Inclusion criteria consisted of studies that specifically focus on women diagnosed with GDM and examine long-term health outcomes, such as cardiovascular disease, T2DM, and metabolic syndrome. Only peer-reviewed articles published in English from any year were considered. Studies conducted in 2023-2024 were included. Additionally, studies that employ quantitative, qualitative, or mixed-methods research designs were included.
Exclusion criteria encompassed studies that do not specifically target women with GDM or fail to report on long-term health outcomes. Case reports, reviews, editorials, and conference abstracts were excluded to maintain a focus on original research. Furthermore, studies not published in English or those lacking rigorous methodological quality, as determined by the assessment tools employed, were omitted from the review. This structured approach helped ensure that the findings of the systematic review were based on relevant and high-quality evidence.
Data Extraction
To guarantee accuracy, the search results were validated with Rayyan (QCRI) [11]. Titles and abstracts found in the search were appraised for relevance using the inclusion and exclusion criteria. Papers that met the inclusion criteria were thoroughly reviewed by the study team. Any differences were settled by consensus. Key study information, including titles, authors, publication year, study location, participant demographics, long-term complications of GDM (CVD & T2D), and main outcomes, were recorded using a predefined data extraction form. An independent evaluation technique was designed to assess the risk of bias.
Data Synthesis Strategy
To give a qualitative review of the research findings and components, summary tables were created utilizing data from pertinent studies. After collecting data for the systematic review, the best way to use the data from the included studies was established.
Risk of Bias Assessment
To assess the study's quality, the Joanna Briggs Institute (JBI) [12] critical review criteria for studies providing prevalence data was applied. This tool consists of nine questions, each with a score of one for positive responses and zero for negative, confusing, or irrelevant responses. Scores below 4, 5 to 7, and 8 or higher were classified as low, moderate, and high quality, accordingly. The researchers separately evaluated the quality of the studies, and any discrepancies were handled by discussion.
Results
Systematic search outcomes
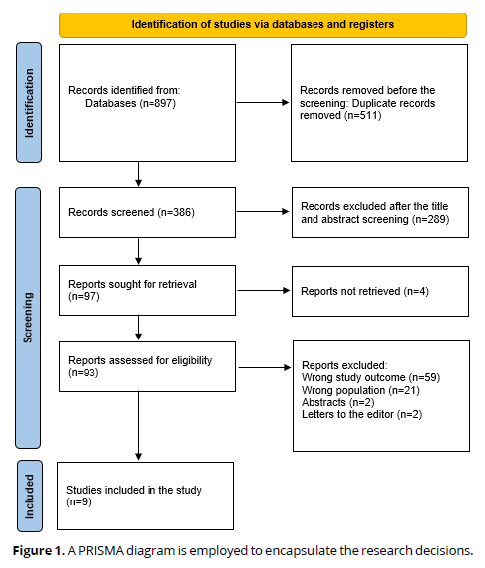
A thorough search of 897 study papers yielded 511 duplicates that were disregarded. After reviewing the titles and abstracts of 386 studies, 289 articles were rejected. Out of the 97 reports that were necessary, 4 were not found. 59 papers were excluded because the study results were inaccurate, 2 were editor's letters, and 2 were abstracts. 21 were disqualified for using the wrong demographic types. The qualifying requirements are met by the nine research publications that comprise this systematic review. A diagram illustrates the process by which the literature was selected in (Figure 1).
Sociodemographics and clinical parameters of the involved participants and studies
(Table 1) summarizes the socio-demographic data from the research articles. Our analysis included nine studies with a total of 39,969 patients diagnosed with GDM. The studies consisted of prospective cohorts [16-20], and retrospective cohorts [13-15, 21]. Geographically, three studies were conducted in the USA [16, 17, 18], two in Israel [14, 15], and one each in Denmark [13], China [19], Finland [20], and France [21].
| Study ID | Study design | Country | Participants (n) | Mean age |
|---|---|---|---|---|
| Nielsen et al., 2024 [13] | Retrospective cohort | Denmark | 20873 | 32.7 ± 5.13 |
| Maor-Sagie et al., 2024 [14] | Retrospective cohort | Israel | 7567 | 32 |
| Naeh et al., 2024 [15] | Retrospective cohort | Israel | 1812 | 33.1 |
| Wang et al., 2023 [16] | Prospective cohort | USA | 1,320 | 28.5 ± 6.6 |
| Wei et al., 2024 [17] | Prospective cohort | USA | 632 | 44 |
| Minhas et al., 2024 [18] | Prospective cohort | USA | 53 | 39.8 ± 5.3 |
| Ying et al., 2024 [19] | Prospective cohort | China | 510 | NM |
| Bakiris et al., 2024 [20] | Prospective cohort | Finland | 271 | 34 ± 5.3 |
| Bullough et al., 2024 [21] | Retrospective cohort | France | 6931 | 32.4 ± 5.7 |
(Table 2) shows the clinical parameters
Study ID |
Follow-up (years) | Condition | Prevalence (%) | Main outcomes | JBI |
|---|---|---|---|---|---|
| Nielsen et al., 2024 [13] | 7.3 | T2DM | 2277 (10.9%) | Regardless of age, education level, or BMI, migrant women were more likely than Danish women to acquire T2DM. However, the risk of T2DM differed greatly based on the country/region of origin. | Low |
| Maor-Sagie et al., 2024 [14] | 4.3 | T2DM | 697 (9.2%) | GDM accelerates the progression to T2DM to varying degrees, depending on the date of diagnosis and baseline risk factors. Early GDM is associated with an increased risk of T2DM, especially in patients without obesity, emphasizing the necessity of taking individual factors into account when assessing T2DM development. | Moderate |
| Naeh et al., 2024 [15] | 3.9 | T2DM | 303 (16.7%) | GDM in multifetal pregnancies is twice as likely to proceed to T2DM after birth as GDM in singleton pregnancies, particularly in obese patients. | Moderate |
| Wang et al., 2023 [16] | 11.8 | T2DM | 216 (16.4%) | GDM was linked to a variety of lipid metabolic changes in the early postpartum period, including several lipid species that were also linked to incident T2D and facilitated the transition from GDM to T2D. | Moderate |
| Wei et al., 2024 [17] | NM | T2DM | 173 (27.4%) | Mean platelet volume was found to be positively linked with the risk of T2DM in women with a previous diagnosis of GDM in the population of the United States. | Moderate |
| Minhas et al., 2024 [18] | 10 | CVD | NR | Developing GDM within the last decade has been correlated with worse cardiac structure/function and arterial endothelial function. | Moderate |
| Ying et al., 2024 [19] | 6.7 | CVD | NR | A history of GDM was not found to be significantly associated with an elevated risk of total cardiac death. | Moderate |
| Bakiris et al., 2024 [20] | 11.9 | CVD | NR | Women who have previously been diagnosed with GDM have greater risk factors for CVD in midlife and are more likely to experience cardiovascular events later in life. | Moderate |
| Bullough et al., 2024 [21] | 5 | CVD | NR | Women having a history of both GH and GDM have a 23-fold higher risk of myocardial infarction during the first 5 years of follow-up. | Moderate |
T2DM
The prevalence of T2DM as a long-term outcome following GDM ranged from 9.2% [14] to 27.4% [17]. Research indicates that women who have experienced GDM face a significantly increased risk of developing T2DM later in life. This risk isn't uniform and can vary based on several factors. For example, migrant women have been shown to have a higher likelihood of transitioning to T2DM compared to women who are native to the same region [13, 14].
Moreover, the timing of the GDM diagnosis and individual health conditions can influence the risk at which T2DM develops. Women who are diagnosed with GDM early in their pregnancy and multiple pregnancies, are at a higher risk of progressing to T2DM [15, 16].
Another important finding is the link between increased mean platelet volume and the risk of T2DM among women with a history of GDM. This could potentially serve as a useful biomarker for identifying those at greater risk, allowing for earlier and more targeted preventive measures [17].
CVD
GDM diagnosed within the last decade has been linked to deteriorated heart structure and function, as well as impaired arterial endothelial function. These changes can potentially contribute to a higher likelihood of CVD in later years [18, 20]. Women with GDM have a 23-fold increased likelihood of experiencing a myocardial infarction within the first five years after their pregnancy, which indicates a strong correlation between these conditions and severe cardiovascular outcomes [21]. Interestingly, one study found that a history of GDM did not significantly increase the risk of total cardiac death [19] (Table 1&2).
Discussion
This review found that the prevalence of T2DM as a long-term outcome following GDM ranged from 9.2% [14] to 27.4% [17]. We found that women who have had GDM have a much higher chance of getting T2DM later in life. Early GDM diagnosis, obesity, and multifetal pregnancies all contribute to an increased risk. Geographic and socioeconomic factors also influence the evolution of T2DM. The strength of the link between GDM and T2DM suggests that more regular assessments and effective therapies for eligible women are required. The American Diabetes Association and other medical organizations urge that women with GDM have a diabetes test 6-12 weeks after giving birth [22]. Despite the emphasis on numerous standards, postpartum testing compliance rates remain low [23, 24].
In line with our results, Vounzoulaki et al. reported that women with a history of GDM appear to be about ten times more likely to develop T2DM than those with a normoglycemic pregnancy. The enormity of this risk emphasizes the significance of intervening to prevent the emergence of T2DM, especially in the early years following pregnancy [25]. Kim et al. also found a greater cumulative incidence of T2DM in the first five postpartum years, which appeared to level in subsequent years [26].
We found that GDM diagnosed during the previous decade has been associated with poor cardiac shape and function, as well as altered arterial endothelial function. These modifications may lead to an increased risk of CVD in later life [18, 20]. Women with GDM are 23 times more likely to have a myocardial infarction within the first five years after their pregnancy, indicating a significant link between these disorders and serious cardiovascular consequences [21]. In recent years, a growing number of observational studies have found that women with GDM have a higher risk of unfavorable cardiovascular outcomes than their non-diabetic counterparts. A previous meta-analysis of nine studies conducted by Karmet et al. found that a history of GDM mellitus was related to a 1.98 times greater risk of future cardiovascular events overall [27]. Xie et al. found that GDM is linked to an increased risk of both general and type-specific cardiovascular and cerebrovascular disorders, which cannot be attributed only to traditional cardiovascular risk factors or diabetes [28].
The clinical consequences of these discoveries are significant. Healthcare practitioners should be aware of the higher long-term risks for women with GDM and conduct extensive postpartum monitoring and preventative care methods. This could include regular glucose tolerance testing, cardiovascular risk assessments, and lifestyle interventions including nutritional counseling and exercise regimens customized to these women's specific requirements. Early detection and management of modifiable risk factors, such as obesity and dyslipidemia, is critical in lowering the prevalence of T2DM and CVD in this population. Furthermore, healthcare institutions should consider including GDM history in routine health records to ensure that women receive adequate follow-up care throughout their lives. Public health initiatives focused on raising awareness about the long-term risks associated with GDM and promoting healthy lifestyle choices could also play a vital role in mitigating these risks.
Strengths and limitations
This review's merits include a thorough evaluation of research from various demographics, which provides a broad perspective on the long-term dangers associated with GDM. The inclusion of metabolic and cardiovascular outcomes deepens our understanding of GDM's diverse influence on women's health. Furthermore, the analysis identifies prospective biomarkers that could be utilized to improve risk classification and clinical decision-making.
However, there are certain limits to consider. The variability in study design, follow-up duration, and outcome measures among the included studies may restrict the comparability of results. Furthermore, the use of retrospective data in some research may introduce recall bias and influence the accuracy of reported results. The lack of specific information on the impact of lifestyle factors such as nutrition and physical exercise further hinders our capacity to completely comprehend the modifiable components of the reported hazards. Future studies should try to overcome these limitations by including more varied cohorts and using standardized procedures to improve the evidence's robustness.
Conclusion
Women having a history of GDM are at a greatly elevated risk of acquiring T2DM and CVD later in life. These findings highlight the importance of providing proactive and customized postpartum care to reduce these risks. Healthcare practitioners should prioritize regular monitoring and preventative interventions for this population, taking into account the numerous risk factors that can impact the progression of GDM to long-term health consequences. Future research should concentrate on discovering the underlying mechanisms and establishing effective ways for early intervention and risk mitigation. By doing so, we can enhance the long-term health outcomes for women with GDM and lessen the burden of chronic diseases in this high-risk group.
References
Sheiner E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention?. Front Clin Diabetes Healthc. 2020;1:546256. Published 2020 Nov 4.
Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes (2015) 6(8):1065–72.
Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet (2009) 373:1773–9.
Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care (2006) 29:2078–83.
Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol (2007) 47:307–12.
Fuchs O, Sheiner E, Meirovitz M, Davidson E, Sergienko R, Kessous R. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch Gynecol Obstet (2017) 295(3):731–6.
Beharier O, Sergienko R, Kessous R, Szaingurten-Solodkin I, Walfisch A, Shusterman E, et al.. Gestational diabetes mellitus is a significant risk factor for long-term ophthalmic morbidity. Arch Gynecol Obstet (2017) 295:1477–82.
Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al.. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care (2008) 31:340–6.
Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al.. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes (2000) 49:2208e11.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International journal of surgery. 2021 Apr 1; 88:105906.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic reviews. 2016 Dec; 5:1-0.
Munn Z, Aromataris E, Tufanaru C, Stern C, Porritt K, Farrow J, Lockwood C, Stephenson M, Moola S, Lizarondo L, McArthur A. The development of software to support multiple systematic review types: the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). JBI evidence implementation. 2019 Mar 1;17(1):36-43.
Nielsen H, Windolf-Nielsen A, Scheuer SH, Damm P, Nybo Andersen AM, Andersen GS, Kragelund Nielsen K. Type 2 Diabetes Risk After Gestational Diabetes According to Country/Region of Origin: A Nationwide Register-based Study. The Journal of Clinical Endocrinology & Metabolism. 2024 Mar 1:dgae113.
Maor-Sagie E, Hallak M, Haggiag N, Naeh A, Toledano Y, Gabbay-Benziv R. Timing of gestational diabetes diagnosis and progression to type 2 Diabetes: A comparative analysis. Diabetes Research and Clinical Practice. 2024 Aug 1; 214:111782.
Naeh A, Maor-Sagie E, Hallak M, Toledano Y, Gabbay-Benziv R. Greater risk of type 2 diabetes progression in multifetal gestations with gestational diabetes: the impact of obesity. American Journal of Obstetrics and Gynecology. 2024 Feb 15.
Wang G, Buckley JP, Bartell TR, Hong X, Pearson C, Wang X. Gestational Diabetes Mellitus, Postpartum Lipidomic Signatures, and Subsequent Risk of Type 2 Diabetes: A Lipidome-Wide Association Study. Diabetes care. 2023 Jun 1;46(6):1223-30.
Wei Y, Lin Y, Huang L, Wang C, Li R. Association between mean platelet volume and the risk of type 2 diabetes mellitus among women with history of gestational diabetes mellitus. BMC Endocrine Disorders. 2024 Dec;24(1):1-0.
Minhas AS, Countouris M, Ndumele CE, Selvin E, Vaught AJ, Gandley R, Hays AG, Ouyang P, Villanueva FS, Bennett WL, Michos ED. Association of gestational diabetes with subclinical cardiovascular disease. JACC: Advances. 2024 Aug 1;3(8):101111.
Ying Q, Xu Y, Zhang Z, Cai L, Zhao Y, Jin L. Gestational diabetes mellitus and risk of long-term all-cause and cardiac mortality: a prospective cohort study. Cardiovascular Diabetology. 2024 Feb 1;23(1):47.
Bakiris E, Luiro K, Jokelainen J, Morin‐Papunen L, Keinänen‐Kiukaanniemi S, Kaikkonen K, Piltonen T, Tapanainen JS, Auvinen J. Women with a history of gestational diabetes mellitus present an accumulation of cardiovascular risk factors at age 46—A birth cohort study. Acta Obstetricia et Gynecologica Scandinavica. 2024 May 9.
Bullough S, Lip GY, Fauchier G, Herbert J, Sharp A, Bisson A, Ducluzeau PH, Fauchier L. The impact of gestational diabetes and gestational hypertension on future cardiovascular events: A nationwide cross-sectional cohort study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2024 Oct 1; 301:216-21.
Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61.
Nielsen JH, Olesen CR, Kristiansen TM, Bak CK, Overgaard C. Reasons for women's non-participation in follow-up screening after gestational diabetes. Women Birth. 2015;28:e157–63.
Yarrington C, Zera C. Health systems approaches to diabetes screening and prevention in women with a history of gestational diabetes. Curr Diab Rep. 2015; 15:114.
Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. bmj. 2020 May 13;369.
Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002; 25:1862-8. 10.2337/diacare.25.10.1862.
Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019; 62:905-14. 10.1007/s00125-019-4840-2.
Xie W, Wang Y, Xiao S, Qiu L, Yu Y, Zhang Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: systematic review and meta-analysis. bmj. 2022 Sep 21;378.