Research Article - (2024) Volume 19, Issue 5
Nebulized Corticosteroids For Acute Asthmatic Attacks A Systematic Review Of Randomized Control Trials
Saleh Farhan M. Alanazi1*, Abdulkarim Ahmed M Alshammari2, Rayan Ahmed Alanazi2, Turki Hani S Alhazmi3, Saud Ghadeer M Alanazi3, Kholoud Obeid H Al Bathaly4, Ahmed Rahil S Alanazi5 and Fahad Wadi S Alanazi5*Correspondence: Saleh Farhan M. Alanazi, Emergency and Critical Care Consultant, Prince Abdulaziz Bin Musaad Hospital, Arar, Saudi Arabia, Email:
2General practitioner, Emergency Department, Prince Abdulaziz Bin Musaad Hospital, Arar, Saudi Arabia
3Medical intern, Northern Border University, Arar, Saudi Arabia
4General Practitioner, Janop Abo Mosa Primary Health Care center, Hafar al-Batin, Saudi Arabia
5General practitioner, Emergency Department, North Medical Tower Hospital, Arar, Saudi Arabia
Received: 25-Sep-2024 Published: 12-Oct-2024
Abstract
Objectives: To evaluate the effectiveness and safety of nebulized corticosteroids in the management of acute asthmatic attacks.
Methods: A thorough search across four databases identified 513 relevant publications. After removing duplicates using Rayyan QCRI and screening for relevance, the search yielded 226 publications, of which 37 fulltext articles were reviewed, and 5 met the eligibility criteria for evidence synthesis.
Results: We included 5 studies with a total of 361 patients (176 in the case group and 185 in the control group) and less than half of them were females 163 (45.2%). Nebulized corticosteroids, more specifically budesonide, are one of the promising treatments in acute asthma exacerbation, especially in children, because of its equal efficacy compared to systemic steroids but with fewer adverse effects. Corticosteroids may improve outcomes when given along with other treatments in adults, but as an intervention in isolation, the outcome is still uncertain. Much larger and standardized studies are required to evaluate the complete potential of nebulized corticosteroids in both children and adults.
Conclusion: Nebulized corticosteroids, more specifically budesonide, are a promising treatment option for acute asthma exacerbation and, in particular, in children because of its equal efficacy as compared to systemic steroids with less adverse effects. Corticosteroids may enhance outcomes when used in combination with other interventions in adults; as an isolated intervention, however, the outcome remains uncertain. Further largescale and standardized studies are necessary to establish the full potential of nebulized corticosteroids both in pediatric and adult populations.
Keywords
Nebulized corticosteroids; Acute asthma exacerbations; Emergency treatment; Systematic review.
Introduction
Respiratory symptoms, reversible airflow restriction, and hyper-responsiveness are all hallmarks of asthma, a chronic inflammatory disease of the airways [1, 2]. In both the developed and developing worlds, it is the most prevalent chronic lung disease. Evidence suggests that its prevalence has risen globally during the past 20 years [3–5].
Every asthmatic patient is susceptible to exacerbations, which are marked by a progressive worsening of coughing, wheezing, chest tightness, and shortness of breath, as well as a reduction in expiratory airflow. This condition has been referred to as acute asthma (AA) or asthma attack. Exacerbations can range in severity from minor to potentially fatal. Although deterioration typically occurs over hours, days, or weeks, some individuals have abrupt and unanticipated increases in airway blockage (over minutes). At least 12 different places worldwide have reported experiencing epidemic asthma, which is the simultaneous occurrence of an abnormally large number of asthma attacks in one region at one time [6].
ED and critical care professionals frequently deal with asthma attack as a medical emergency. Adolescents and young adults are the age groups most likely to seek treatment at the emergency department (ED), with asthma ranking as the eleventh most common ED diagnosis in the US [7]. Hospitalization and ED visits for asthma attack are twice as common among women as among males [8].
Management is based on how severe asthma flare-ups are. The objectives of treatment can be summed up as follows: reducing airway inflammation and preventing future relapses by early systemic corticosteroid administration; relieving airflow obstruction with repeated administration of rapid-acting inhaled bronchodilators (such as -agonists and anticholinergics); and maintaining adequate arterial oxygen saturation with supplemental oxygen [9].
While systemic corticosteroids have long been the standard treatment for acute asthma attacks due to their anti-inflammatory properties, they come with the potential for significant side effects, particularly with repeated or long-term use [15]. In recent years, nebulized corticosteroids have emerged as a promising alternative. Administered directly into the lungs, nebulized corticosteroids may provide a more targeted approach, reducing systemic absorption and potentially lowering the risk of side effects associated with systemic administration [17]. However, despite their increasing use in clinical settings, the efficacy and safety profile of nebulized corticosteroids in treating acute asthmatic attacks remain under debate. Given the growing interest in optimizing asthma management while minimizing adverse effects, a systematic review of randomized controlled trials (RCTs) assessing the outcomes of nebulized corticosteroids is crucial to determine their role in acute asthma treatment. This systematic review aims to evaluate the effectiveness and safety of nebulized corticosteroids in the management of acute asthmatic attacks
Methods
Search strategy
The systematic review adhered to the PRISMA and GATHER criteria. A thorough search was undertaken to locate relevant studies on the effectiveness and safety of nebulized corticosteroids in the management of acute asthmatic attacks. The reviewers looked at four electronic databases: PubMed, Cochrane, Web of Science, and SCOPUS. Studies published between 2019-2024 were included. We uploaded all of the titles and abstracts identified through electronic searches into Rayyan, removing any duplicates. All texts from papers that met the inclusion criteria based on title or abstract were collected and thoroughly inspected. Two reviewers independently evaluated the appropriateness of the extracted publications and resolved any contradictions through discussion.
Study population-selection
The PICO (Population, Intervention, Comparison, and Outcome) factors were implemented as inclusion criteria for our review: (i) Population: Patients with acute asthmatic attacks, (ii) Intervention: Nebulized corticosteroids, (iii) Comparator: Other anti-asthmatic medications, (iv) Outcome: Effectiveness and safety of nebulized corticosteroids. Only primary investigations studying the administration of biological treatment to pustular psoriasis were included.
Data extraction
Two unbiased reviewers retrieved data from studies that met the inclusion criteria in a consistent and established format. The following information was retrieved and recorded: (i) First author (ii) Year of publication, (iii) Study design, (iv) Country, (v) Sample size, (vi) Gender, (vii) Age (viii) Population type, (ix) Intervention/ dosage, (x) Main outcomes (effectiveness and safety).
Quality review
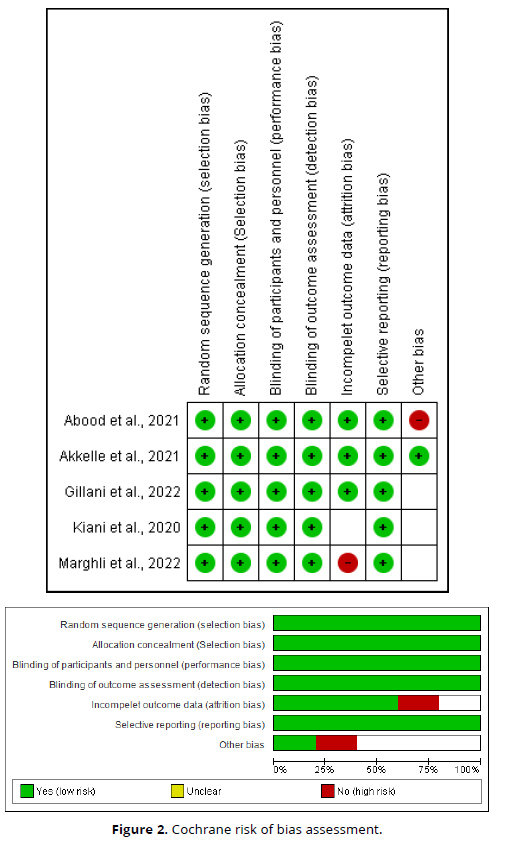
The Cochrane Risk of Bias Instrument [10] was used to conduct a critical appraisal of the identified RCTs. This tool evaluates the risk of bias in seven fields: arbitrary sequence generation, allocation secrecy, blinding of participants and employees, blinding of outcome evaluation, inadequate outcome data, selective reporting, and additional bias sources. The risk of bias in each of these domains was classified as low, unclear, or high.
Results
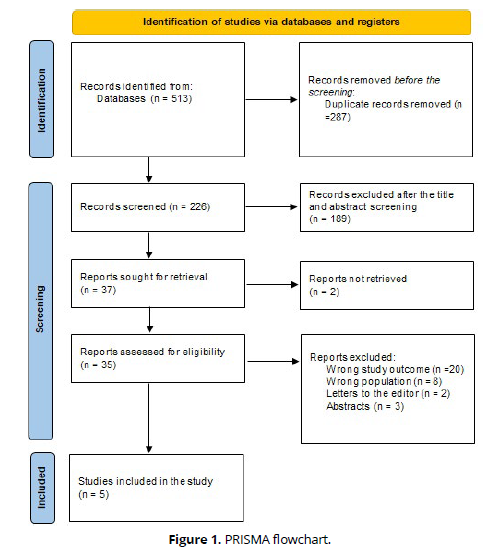
The specified search strategy yielded 513 publications (Figure 1). After removing duplicates (n = 287), 226 articles were evaluated based on title and abstract. Of these, 189 failed to satisfy eligibility criteria, leaving just 37 full-text articles for comprehensive review. A total of 5 satisfied the requirements for eligibility with evidence synthesis for analysis (Figure 1).
Sociodemographic and clinical outcomes
We included 5 studies with a total of 361 patients (176 in the case group and 185 in the control group) and less than half of them were females 163 (45.2%). All of the included studies RCTs [12-16]. One study was implemented in Iran [12], one in Tunisia [13], one in Turkey [14], one in Pakistan [15], and one in Iraq [16].
In adults, the use of nebulized corticosteroids, specifically budesonide, appears to offer potential benefits in managing acute asthma attacks. Nebulized budesonide may contribute to hastening recovery by improving respiratory flow and potentially shortening hospital stays when included as part of a comprehensive treatment regimen. This regimen often includes oxygen, systemic corticosteroids, and short-acting beta agonists (SABA). However, in emergency settings, nebulized corticosteroids, when used alongside other medications like hydrocortisone, may not consistently provide additional benefits compared to the effects of hydrocortisone alone [12, 13].
In children, nebulized budesonide has shown promising results, particularly in cases of moderate asthma attacks. Studies indicate that low-dose nebulized budesonide may be just as effective as systemic steroids in reducing the severity of symptoms and hospital stay length. Moreover, when combined with nebulized salbutamol, budesonide significantly improves clinical outcomes and reduces emergency room stays, providing a substantial benefit in managing acute exacerbations in pediatric patients [14-16] (Table 1, Figure 2).
Study ID |
Country |
Study design |
Sociodemographic |
Population type |
Intervention |
Main outcomes |
Kiani et al., 2020 [12]
|
RCT
|
Iran
|
Cases: 55 |
Adults
|
Nebulized budesonide (0.5 mg/2 ml) |
Nebulized budesonide may hasten recovery, improve respiratory flow, and shorten hospital stays when added to the primary therapy regimen for acute asthma attacks that also includes oral oxygen, SABA, and systemic corticosteroids |
Marghli et al., 2022 [13]
|
RCT
|
Tunisia
|
Cases: 23 |
Adults
|
Budesonide (0.5mg) at 20, 40, 60 and 120 min
|
When treating adults' acute asthma in the emergency department, nebulized budesonide and hydrocortisone hemisuccinate do not have any more effects than hydrocortisone alone. |
Akkelle et al., 2021 [14]
|
RCT
|
Turkey
|
Cases: 28 |
Children
|
Nebulized budesonide 500 µg / dose
|
In terms of effectiveness and length of hospital stay, low-dose nebulized budesonide treatment is just as successful as systemic steroids in treating moderate asthma attacks in children. |
Gillani et al., 2022 [15] |
RCT |
Pakistan |
Cases: 20 |
Children |
NS |
When treating acute exacerbation asthma, nebulized steroids were more beneficial and effective than systemic steroids in terms of lowering hospital stays and illness severity. |
|
|
|
Controls: 20 |
|
|
|
Abood et al., 2021 [16]
|
RCT
|
Iraq
|
Cases: 52 |
Children
|
2.5 mg of salbutamol solution was combined with 500 μg of budesonide suspension.
|
When nebulized budesonide and salbutamol were used together to treat asthmatic children experiencing mild to severe acute exacerbations, the children's clinical condition significantly improved and their length of stay in the emergency room was shortened in comparison to those receiving nebulized salbutamol alone. |
NS=Not-Specified
Table 1. Outcome measures of the included studies.
Discussion
This systematic review demonstrated that in adults, nebulized budesonide may enhance recovery, improve respiratory flow, and shorten hospital stays when added to standard asthma treatment; however, any additional benefit beyond hydrocortisone administered alone remains uncertain. Similarly, Kearns et al. validated the effectiveness of ICS for patients with acute asthma who visit the emergency department. When treating moderate-to-severe asthma exacerbations in the emergency department, there is some evidence that large doses of ICS, when combined with systemic corticosteroids, lower the likelihood of hospitalization [17]. Krishnan et al. also found that Oral and ICS combinations may reduce the chance of relapse after hospital or emergency department discharge [18].
It is interesting to consider the possible mechanisms through which ICS may have acute effects on asthmatic patients experiencing a severe exacerbation. 30 minutes after inhaling fluticasone [19] and budesonide [20], ICS temporarily decrease airway mucosal blood flow in asthmatic patients by acutely suppressing airway hyperperfusion. This short-lived nongenomic action has a quick onset and is linked to the plasma membrane rather than glucocorticoid receptors [21]. The extra effectiveness of ICS when given in addition to SCS in the acute context may be explained by the fact that the genomic effects of corticosteroids take at least 6 to 12 hours to fully manifest [22].
This review also found that nebulized budesonide was effective in treating a moderate asthma attack in children, especially in combination with nebulized salbutamol, offering a clinically comparable response to systemic steroids with regard to mean symptom score and duration of hospital stay. These results highlight nebulized corticosteroids as a potentially useful alternative in the management of acute asthma, especially in pediatric patients. In the line with our results, Castro‐Rodriguez et al. reported that adding budesonide to systemic corticosteroid reduces length of stay and improves acute asthma score in children in an emergency department setting, but it has no effect on hospitalization rate when compared to systemic corticosteroid alone [23]. Direkwattanachai et al. also found that in order to treat acute asthma flare-ups of any severity, nebulized corticosteroids may be a useful treatment choice. Nebulized budesonide is the corticosteroid of choice [24].
This would mean that nebulized corticosteroids could replace systemic corticosteroids in clinical practice, especially in children, where the avoidance of systemic adverse effects is desirable. Such a mode of treatment might prove particularly useful in the emergency setting, where quick improvement and, consequently, shorter hospitalization may actually mean less cost and better patient throughput. In adults, nebulized corticosteroids may confer some benefits; however, the overall role of these agents, in addition to other modalities such as hydrocortisone, is not well defined and may not be consistently advantageous. In general, nebulized corticosteroids seem promising for both pediatric and adult populations, but the most solid support in favor is for pediatric use.
The aim of this review is to emphasize the role of nebulized corticosteroids in acute asthma management by systematically reviewing the best evidence available, represented by RCT. Because the review has focused on RCTs, this makes the evidence quite robust with minimal biases, hence more reliable. The inclusion of different studies across a wide age spectrum improves the external validity of nebulized corticosteroid efficacy for both adults and children.
Considering these strengths, some limitations should be considered. The heterogeneity of treatment protocols concerning different medications, dosages, and co-administered medication across studies complicates comparing results, at least in adults. Secondly, sample sizes of some of the included studies were rather small, which may reduce the generalizability of findings. Lastly, regional differences concerning nebulized corticosteroid availability in clinical practice and differences in healthcare practice might further restrict the generalization of these findings to larger populations.
Conclusion
Nebulized corticosteroids, more specifically budesonide, are a promising treatment option for acute asthma exacerbation and, in particular, in children because of its equal efficacy as compared to systemic steroids with less adverse effects. Corticosteroids may enhance outcomes when used in combination with other interventions in adults; as an isolated intervention, however, the outcome remains uncertain. Further large-scale and standardized studies are necessary to establish the full potential of nebulized corticosteroids both in pediatric and adult populations.
References
Global strategy for asthma management and prevention. NIH Publication 02–3659, 2002. Available at: http:// www.ginasthma.com. Accessed October 30, 2024
National Asthma Education and Prevention Program. Expert panel report 2: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, 1997; publication No. 55–4051.
Worldwide variations in the prevalence of asthma symptoms: the international study of asthma and allergies in childhood (ISAAC). Eur Respir J 1998; 12:315–335.
Mannino DM, Homa DM, Pertowski CA, et al. Surveillance for asthma: United States, 1960–1995. MMWR Morb Mortal Wkly Rep 1998; 47:1–27.
Self-report asthma prevalence among adults–United States 2000. MMWR Morb Mortal Wkly Rep 2001; 50:682–686.
Anto´ JM, Sunyer JM. Epidemic asthma. In: Holgate ST, Boushey HA, Fabbri LM, eds. Difficult asthma. London, UK: Martin Dunitz Ltd, 1999; 333–340.
Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993–1994. Adv Data 1996; 277:1
Trawick DR, Holm C, Wirth J. Influence of gender on rates of hospitalization, hospital course, and hypercapnea in highrisk patients admitted for asthma: a 10-year retrospective study at Yale-New Haven hospital. Chest 2001; 119:115–119.
Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004 Mar 1;125(3):1081-102.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011 Oct 18;343.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015 Dec;4:1-9.
Kiani A, Razavi F, Farahani M, Valizadeh J, Bandegani N, Emami H, Abedini A. Nebulized Budesonide in the Patients with Acute Asthma Exacerbation: A Randomized Clinical Trial. Biomedical and Biotechnology Research Journal (BBRJ). 2020 Apr 1;4(2):173-6.
Marghli S, Bouhamed C, Sghaier A, Chebbi N, Dlala I, Bettout S, Belkacem A, Kbaier S, Jerbi N, Bellou A. Nebulized budesonide combined with systemic corticosteroid vs systemic corticosteroid alone in acute severe asthma managed in the emergency department: a randomized controlled trial. BMC Emergency Medicine. 2022 Jul 23;22(1):134.
Akkelle BS, Aydogan M, Siraneci R. Comparison of the efficacy of nebulized budesonide and systemic steroids in children admitted to the emergency service with acute asthma attacks.
Gillani S, Qazi GA, Ibrahim SK, Bibi S, Farooq AH. Comparison of Nebulized vs Systemic Corticosteroids for Management of Children Presenting with Acute Exacerbation of Asthma. Pakistan Journal of Medical & Health Sciences. 2022 Apr 5;16(02):938-.
Abood HA, Al-Musawi ZM, Hussein AM, Hameed RM. Effects of nebulized budesonide plus salbutamol and nebulized salbutamol monotherapy on mild to moderate acute exacerbation of asthma in children: A comparative study. clinical trials. 2021 Dec 1;8:10.
Kearns N, Maijers I, Harper J, Beasley R, Weatherall M. Inhaled corticosteroids in acute asthma: a systemic review and meta-analysis. The Journal of Allergy and Clinical Immunology: In Practice. 2020 Feb 1;8(2):605-17.
Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: corticosteroid therapy for adults with acute asthma. The American journal of medicine. 2009 Nov 1;122(11):977-91.
Kumar SD, Brieva JL, Danta I, Wanner A. Transient effect of inhaled fluticasone on airway mucosal blood flow in subjects with and without asthma. Am J Respir Crit Care Med 2000; 161:918-21.
Paredi P, Kharitonov SA, Barnes PJ. Correlation of exhaled breath temperature with bronchial blood flow in asthma. Respir Res 2005; 6:15.
Duval D, Durant S, Homo-Delarche F. Non-genomic effects of steroids interactions of steroid molecules with membrane structures and functions. Biochim Biophys Acta1983; 737:409-442.
Rodrigo G, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma: an evidence-based evaluation. Chest 1999; 116:285-95.
Castro‐Rodriguez JA, Pincheira MA, Escobar‐Serna DP, Sossa‐Briceño MP, Rodriguez‐Martinez CE. Adding nebulized corticosteroids to systemic corticosteroids for acute asthma in children: A systematic review with meta‐analysis. Pediatric Pulmonology. 2020 Oct;55(10):2508-17.
Direkwattanachai C, Aksilp C, Chatchatee P, Jirapongsananuruk O, Kamalaporn H, Kamchaisatian W, Lochindarat S, Ngamtrakulpanit L, Poachanukoon O, Trakultivakorn M, Teeratakulpisarn J. Practical considerations of nebulized corticosteroid in children with acute asthmatic exacerbation: a consensus. Asian Pac J Allergy Immunol. 2019;10.