Research - (2024) Volume 19, Issue 4
*Correspondence: Yasir Wadi Alngzi Alanazi, Senior registrar of family medicine, Ministry of defence, Tabuk, Saudi Arabia, Email:
Abstract
Background: Inflammatory Bowel Disease (IBD) can significantly impact a patient's quality of life, and depression is a common mental health concern in individuals with chronic illnesses.
Objectives: To systematically analyze existing research on the link between IBD and depression, focusing on prevalence and potential influencing factors. Methods: We conducted a systematic search of electronic databases like PubMed, MEDLINE, Science Direct, and Scopus. Two independent reviewers screened and extracted data from eligible studies.
Results: Nine studies including 7925 participants in total and more than half of them 4210 (53.1%) were females-were included in our data. The prevalence of depression among IBD patients ranged from 7.5% to 53.2%, with a total prevalence of 2161 (27.3%). The increased incidence of depression in the IBD population was significantly associated with female gender, educational attainment, history of smoking, IBD-related operations, having a co-occurring diagnosis of hypertension or cerebrovascular injury, disease activity, and more corticosteroid use.
Conclusion: The prevalence of depression in IBD patients was relatively high. Many risk factors were documented to increase the incidence of depression in IBD such as female gender and disease activity. The prevalence of depression in IBD patients was relatively high. Many risk factors were documented to increase the incidence of depression in IBD such as female gender and disease activity
Keywords
Inflammatory Bowel Disease (IBD); Crohn's disease; Ulcerative colitis; Depression; Depressive symptoms; Systematic review.
Introduction
IBD is a chronic condition that affects the digestive tract, causing inflammation, ulcers, and damage to the lining of the intestines. There are two main types of IBD, Crohn's disease and ulcerative colitis, both of which can cause a range of symptoms including abdominal pain, diarrhea, fatigue, and weight loss. IBD can have a significant impact on physical health, but recent research has also shown a strong link between IBD and mental health, particularly in terms of depressive symptoms in both children and adults [1].
The relationship between IBD and depressive symptoms is complex and multifaceted. One of the key factors contributing to this relationship is the chronic nature of IBD. Living with a chronic illness can be emotionally taxing, as individuals must cope with the physical symptoms of the disease, as well as the impact it can have on their day-to-day lives. The unpredictable nature of IBD, with periods of remission and flare-ups, can also contribute to feelings of anxiety and depression [2].
In addition to the emotional toll of living with IBD, there are also biological factors that may play a role in the development of depressive symptoms. For example, researchers have found that inflammation in the body, which is a hallmark of IBD, can trigger changes in the brain that are linked to depression. Inflammation is thought to affect neurotransmitters, such as serotonin and dopamine, which are involved in regulating mood. This may explain why individuals with IBD are more likely to experience depressive symptoms compared to the general population [3].
Furthermore, the impact of depressive symptoms on individuals with IBD can be significant. Depression can exacerbate physical symptoms of the disease, leading to poorer quality of life and increased healthcare utilization. It can also have a negative impact on treatment adherence, as individuals may be less motivated to follow their prescribed treatment plan. In turn, this can lead to worsening of IBD symptoms and overall disease progression [4].
Children and adolescents with IBD may be particularly vulnerable to the effects of depressive symptoms. Studies have shown that young patients with IBD are at increased risk of developing depression compared to their healthy peers. This can be due to a variety of factors, such as the challenges of managing a chronic illness during a crucial stage of development, as well as the social and emotional impact of living with a stigmatizing condition. It is important for healthcare providers to screen for and address depressive symptoms in children and adolescents with IBD, in order to prevent long-term negative outcomes [5].
There are effective ways to manage depressive symptoms in individuals with IBD. Treatment options may include therapy, medication, lifestyle changes, and support groups. It is essential for healthcare providers to take a holistic approach to care, addressing both the physical and emotional aspects of the disease. By treating depressive symptoms alongside IBD, individuals can experience improved quality of life and better disease outcomes [6].
IBD is a chronic condition impacting both children and adults. Depression is a prevalent mental health issue with serious consequences. Understanding the link between IBD and depression can improve treatment approaches for better overall health. Children and adults with IBD experience various challenges that might contribute to depression. However, the exact relationship between these conditions remains unclear. To systematically evaluate existing research and determine the association between IBD and depressive symptoms in both children and adults.
Study Objectives
- To identify and critically appraise relevant studies on IBD and depression in children and adults.
- To analyze the data and estimate the prevalence of depressive symptoms in IBD patients.
- To explore potential factors influencing the relationship between IBD and depression (e.g., disease activity, age of onset).
- To compare findings between children and adults with IBD to identify potential age-related differences.
Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [7] to evaluate the relationship between IBD and depressive symptoms. A comprehensive search was conducted across electronic databases like PubMed, Web of Science, SCOPUS, and Science Direct. The search strategy combined keywords related to IBD (e.g., Crohn's disease, ulcerative colitis) and depression in children and adults. Two independent reviewers screened the retrieved titles and abstracts against the predefined inclusion and exclusion criteria. Studies meeting the criteria underwent full-text review for data extraction and quality assessment using standardized tools.
Eligibility Criteria
Inclusion criteria
- Studies investigating the relationship between IBD (Crohn's disease or Ulcerative colitis) and depressive symptoms in children or adults.
- Studies using validated depression measures (e.g., Beck Depression Inventory, Children's Depression Inventory).
- Studies published in English within a designated timeframe (e.g., past 10 years).
- Studies with clear differentiation between case (IBD patients) and control groups (healthy individuals).
Exclusion criteria
- Studies focusing on other mental health conditions besides depression (e.g., anxiety).
- Studies solely based on case reports, editorials, or conference abstracts.
- Studies with methodological flaws that hinder data analysis (e.g., unclear methodology, significant bias).
- Studies not separating data for children and adults (if age-related differences are objective).
Data Extraction
To ensure a comprehensive and unbiased review, retrieved titles and abstracts were screened against the defined inclusion and exclusion criteria using Rayyan (QCRI) software [8]. Studies meeting the criteria underwent full-text review by the research team. Any disagreements regarding study selection were resolved through discussion and consensus. Key information was extracted using a standardized data collection form, including author details, publication year, study location, participant demographics, (age and gender ), potential confounding factors, and main outcomes. Additionally, a tool will be developed to assess the risk of bias within the selected studies.
Data Synthesis Strategy
In order to provide a qualitative evaluation of the research findings and components, summary tables were generated using data extracted from relevant studies. Once the data collection for the systematic review is complete, the optimal approach for utilizing the data from the included studies was determined.
Risk of Bias Assessment
For evaluating the study's quality, the Joanna Briggs Institute (JBI) [9] critical assessment criteria for studies reporting prevalence data were employed. This tool comprises nine questions, with positive responses assigned a score of 1 and negative, unclear, or irrelevant responses receiving a score of 0. Scores below 4, between 5 and 7, and above 8 will be classified as low, moderate, and high quality, respectively. Researchers independently assessed the quality of the studies, and any disagreements were resolved through discussion.
Results
Search results
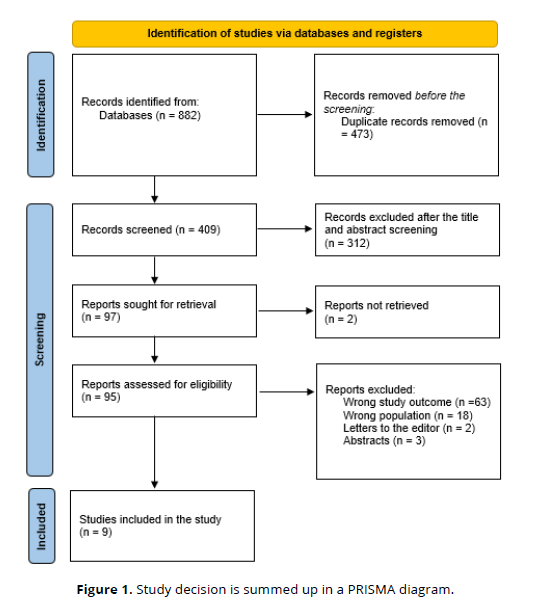
After 473 duplicates were removed, a total of 882 study papers were found through a systematic search. After 409 studies had their titles and abstracts evaluated, 312 papers were discarded. Two articles were not located out of the 97 reports that were required to be retrieved. 95 papers were screened for full-text assessment; 63 were rejected because the study results were wrong, 18 because the population type was inaccurate, 2 articles were editor's letters, and 3 were abstracts. Nine research publications in this systematic review satisfied the requirements for eligibility. An overview of the procedure used to choose the research is illustrated in (Figure 1).
Sociodemographic features of the comprised studies
The research publications' sociodemographic information is displayed in Table 1. Nine studies including 7925 participants in total and more than half of them 4210 (53.1%) were females—were included in our data. Seven studies were cross-sectional studies [10, 12, 14-18], one was retrospective cohorts [11], and one was prospective cohorts [13]. Three studies were conducted in the USA [11, 16, 18], two in Switzerland [13, 17], one in Iran [10], one in Oman [12], and one in Montenegro [14].
Clinical outcomes
The clinical features are displayed in Table (2). Seven studies used the 9-item Patient Health Questionnaire (PHQ-9) [11-17] to diagnose depression, and only two studies used Beck's Depression Inventory (BDI) [10] and the geriatric depression scale (GDS) [18]. The prevalence of depression among IBD patients ranged from 7.5% [13] to 53.2% [17], with a total prevalence of 2161 (27.3%).
The increased incidence of depression in the IBD population was significantly associated with female gender [10, 12], educational attainment [10, 18], history of smoking [10], IBD-related operations [10, 12], having a co-occurring diagnosis of hypertension or cerebrovascular injury [12], disease activity [10, 14, 15, 17, 18], and more corticosteroid use [14, 18]. (Table 1, Table 2).
| Study | Study design | Country | Participants | Mean age | Females (%) |
|---|---|---|---|---|---|
| Joukar et al., 2023 [10] | Cross-sectional | Iran | 164 | 38.5 (14.7) | 81 (49.4%) |
| Jordan et al., 2022 [11] | Retrospective cohort | USA | 90 | 44.47 (13.54) | 42 (46.7%) |
| Al-Aamri et al., 2022 [12] | Cross-sectional | Oman | 201 | 36.2 (9.1) | 103 (51.2%) |
| Jordi et al., 2021 [13] | Prospective cohort | Switzerland | 2255 | 42.2 | 1172 (51.9%) |
| Smolovic et al., 2021 [14] | Cross-sectional | Montenegro | 90 | NM | 49 (54.4%) |
| Wilkinson et al., 2019 [15] | Cross-sectional | UK | 104 | 44 | 52 (50%) |
| Kochar et al., 2018 [16] | Cross-sectional | USA | 4,314 | 41 (15) | 2310 (53.5%) |
| Geiss et al., 2018 [17] | Cross-sectional | Switzerland | 348 | 38 | 181 (52%) |
| Long et al., 2014 [18] | Cross-sectional | USA | 359 | 70 (4.5) | 223 (62.1%) |
| Study | Depression scale | Prevalence of depression | Main outcomes | JBI |
|---|---|---|---|---|
| Joukar et al., 2023 [10] | BDI | 64 (39%) | Compared to men, women experienced more active IBD and depression. Only income was found to differ between the groups with active and inactive IBD (P<0.05), while the prevalence of no, mild, and moderate depression among individuals was significantly different in terms of autoimmune illness, educational attainment, history of smoking, and IBD-related operations. | Moderate |
| Jordan et al., 2022 [11] | PHQ-9 | 24 (25.3%) | Compared to earlier reports for all patients with IBD, the rates of depression among minority patients—mostly African Americans—with IBD were greater. More than 40% of people had mild to severe depression. | Moderate |
| Al-Aamri et al., 2022 [12] | PHQ-9 | 47 (23.4) | An elevated incidence of depressive symptoms is linked to being female, having a history of surgical stoma, and having a co-occurring diagnosis of hypertension and cerebrovascular injury. | Moderate |
| Jordi et al., 2021 [13] | HADS | 168 (7.5%) | Type D showed weak independent relationships with the prognosis of IBD and was highly correlated with depressive symptoms. | Moderate |
| Smolovic et al., 2021 [14] | PHQ-9 | 18 (20%) | Relationship status, thiopurine and corticosteroid use, and illness activity were all statistically substantially correlated with DP. | High |
| Wilkinson et al., 2019 [15] | PHQ-9 | 26 (25%) | Higher rates of depression in individuals with active IBD may be explained by a relationship between disease activity and a reduction in positive biases in emotional perception. | Moderate |
| Kochar et al., 2018 [16] | PHQ-9 | 1548 (35.9%) | Patients with baseline depression who were CD patients were more likely to relapse after controlling for gender, remission, and disease activity. | Moderate |
| Geiss et al., 2018 [17] | PHQ-9 | 185 (53.2%) | It was found that the only significant risk factor for depression in CD patients was clinical disease activity. | High |
| Long et al., 2014 [18] | GDS | 81 (22.5%) | Depression was linked to lower levels of exercise (< 0.001), more corticosteroid use (< 0.01), and lower levels of education (p = 0.001). The disease activity was higher in those with depression for both ulcerative colitis (UC) and CD. | Moderate |
Discussion
Research from numerous nations shows that IBDs are becoming more common, particularly in teenagers. In the first 20 years of life, about 25% of patients with IBD receive a diagnosis [19]. The greatest yearly incidence of IBDs in Europe, according to the M'Koma, was 24.3 per 100,000 person-years for UC and 12.7 per 100,000 person-years for CD [20]. Compared to the general population, individuals with IBD have a higher incidence of depressive disorders. Coexisting depression may be linked to a worse outcome from treatment and predicts a more unfavorable course of the illness [21].
This review investigated the recently implemented literature regarding the incidence of depression in IBD patients. We found that the prevalence of depression among IBD patients ranged from 7.5% [13] to 53.2% [17], with a total prevalence of (27.3%). Depression has become more common during the past ten years, particularly among young adults [22]. Depression and inflammation are related, and this relationship is mediated by the peripheral immune system and substantial cytokine signaling [23]. There are indications that intestinal inflammation and the brain are connected in both directions [24, 25]. According to animal models, depression and bowel inflammation are related, inducing colitis alters the brain, and the gut microbiota may influence how the brain and intestinal inflammation interact [26].
The "biopsychosocial" approach to medicine has put a lot of attention on the connection between emotional variables and gastrointestinal disorders. One of the four aspects of the inflammatory bowel disease questionnaire (IBDQ) [27], which was created in 1989, is emotional variables. Anxiety and depression are widespread health issues that frequently coexist with disease [28]. Early depression is frequently brought on by stress [29]. In IBD, these three related variables are typically present together.
A considerably higher risk of CD and UC was found in depressed patients, according to a study of research based on UK electronic medical records that looked into 403,665 cases of depression with 532,986 people who had no prior history of depression [30]. Depression has been linked to the emergence of symptoms resembling irritable bowel syndrome in IBD patients who are already in remission [31].
IBD-related depression has a dual etiology involving somatic and environmental components. IBD has been proposed to increase patients' feelings of insecurity and change their psychological attachment style at the level of external circumstances, which can cause chronic stress and negatively affect their mental health [32]. Patients may experience neurological dysfunction as a result of the pathogenic effects of IBD at the somatic level. As our understanding of gut-brain interactions grows, conditions including irritable bowel syndrome and IBD that manifest clear signs of gut-brain dysfunction may now be classified as disorders of "gut-brain interaction (DGBI)" [33].
We found that the increased incidence of depression in the IBD population was significantly associated with female gender [10, 12], educational attainment [10, 18], history of smoking [10], IBD-related operations [10, 12], having a co-occurring diagnosis of hypertension or cerebrovascular injury [12], disease activity [10, 14, 15, 17, 18], and more corticosteroid use [14, 18]. Bommena et al. reported that Among people with IBD and the general population, depression affects women more severely. It's interesting to note that women with IBD frequently seek out and are more responsive to information about depression. They talk about how studies on depression in people with IBD need to be gender-based [34].
Among people with IBD and the general population, depression affects women more severely. It's interesting to note that women with IBD frequently seek out and are more responsive to information about depression. They talk about how studies on depression in people with IBD need to be gender-based [35, 36].
Strengths and limitations
This study included recent evidence about the interrelationship between depression and IBD. The authors only included the studies that used validated depression scales for assessment which may be a strength in our review. Additionally, some data were gathered retroactively, which means that some essential clinical details might have been missed. Because of the necessary criteria, diagnosing depression in an IBD patient may be challenging. Certain symptoms, including a shift in weight, that are typical of sadness could be the result of IBD's direct impact on the body.
Conclusion
IBDs are recurrent, chronic inflammatory disorders of the gastrointestinal tract that have an erratic course and uncertain cause. The prevalence of depression in IBD patients was relatively high. Many risk factors were documented to increase the incidence of depression in IBD such as female gender and disease activity. In order to validate these findings, provide additional clarity on the temporal relationship between exposures and outcomes, and determine whether preventative strategies could be beneficial, more thorough cohort studies are required. Further research is necessary to clarify the pathways relating to mood disorders, depression, and the onset of IBD. To further understand the relationship between mood disorders and gut inflammation, as well as if unique genetic susceptibilities may influence this relationship, more creative research is required. Future studies on mechanisms and preventive techniques may be prompted and guided by our findings.
References
Sălcudean, A.; Nan, A.G.; Bodo, C.R.; Cosma, M.C.; Strete, E.G.; Lica, M.M. Association between Childhood Onset Inflammatory Bowel Disease and Psychiatric Comorbidities in Adulthood. Diagnostics2023, 13, 1868. https://doi.org/10.3390/diagnostics13111868
Ali H, Pamarthy R, Bolick NL, Lambert K, Naseer M. Relation between inflammatory bowel disease, depression, and inpatient outcomes in the United States. Proc (Bayl Univ Med Cent). 2022;35(3):278-283. Published 2022 Jan 31. doi:10.1080/08998280.2022.2028344
Mrakotsky C, Forbes PW, Bernstein JH, Grand RJ, Bousvaros A, Szigethy E, et al. Acute cognitive and behavioral effects of systemic corticosteroids in children treated for inflammatory bowel disease. Journal of the International Neuropsychological Society : JINS. 2013;19(1):96–109.
Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4(Suppl 1):S94–8
Nicholas DB, Otley A, Smith C, Avolio J, Munk M, Griffiths AM. Challenges and strategies of children and adolescents with inflammatory bowel disease: a qualitative examination. Health Qual Life Outcomes. 2007;5:28.
Knowles SR, Wilson JL, Connell WR, Kamm MA. Preliminary examination of the relations between disease activity, illness perceptions, coping strategies, and psychological morbidity in Crohn’s disease guided by the common sense model of illness. Inflammatory bowel diseases. 2011;17(12):2551–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International journal of surgery. 2021 Apr 1;88:105906.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Systematic reviews. 2016 Dec;5:1-0.
Munn Z, Aromataris E, Tufanaru C, Stern C, Porritt K, Farrow J, Lockwood C, Stephenson M, Moola S, Lizarondo L, McArthur A. The development of software to support multiple systematic review types: the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). JBI evidence implementation. 2019 Mar 1;17(1):36-43.
Joukar F, Asgharnezhad M, Faraji N, Zeinali T, Hojati A, Maveddati S, Sepehrimanesh M, Isanazar A, Mansour-Ghanaei A, Samadi A, Mansour-Ghanaei F. The Association Between Depression and Activity of Inflammatory Bowel Disease. Caspian Journal of Neurological Sciences. 2023 Apr 10;9(2):102-7.
Jordan A, Mills K, Sobukonla T, Bredy S, Kelly A, Flood M. Depression rates among African American inflammatory bowel disease patients at a large safety net hospital. Colorectal Disease. 2022 Dec;24(12):1550-5.
Al-Aamri H, Al-Huseini S, Chan MF, Al Saadi A, Al-Sibani N, Al-Dughaishi Z, Al-Alawi M. Clinical predictors of depression among patients with inflammatory bowel diseases: a cross-sectional analytical study from Oman. Oman Medical Journal. 2022 Mar;37(2): e352.
Jordi SB, Botte F, Lang BM, Greuter T, Krupka N, Auschra B, Schreiner P, Sulz MC, Biedermann L, von Känel R, Rogler G. Type D personality is associated with depressive symptoms and clinical activity in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2021 Jul;54(1):53-67.
Smolovic B, Lukic M, Bojovic S, Vukovic MN. Inflammatory bowel disease and depressive symptoms: the prevalence and factors associated with depression in patients with inflammatory bowel disease on intravenous biological therapy-single center experience. European Review for Medical & Pharmacological Sciences. 2021 Jun 1;25(11).
Wilkinson B, Trick L, Knight A, Valton V, Goodhand J, Kennedy NA, Heerasing N, Ahmad T, Bland A, Elliott R, Roiser JP. Factors associated with depression in people with inflammatory bowel disease: the relationship between active disease and biases in neurocognitive processing. Neurogastroenterology & Motility. 2019 Aug;31(8):e13647.
Kochar B, Barnes EL, Long MD, Cushing KC, Galanko J, Martin CF, Raffals LE, Sandler RS. Depression is associated with more aggressive inflammatory bowel disease. Official journal of the American College of Gastroenterology| ACG. 2018 Jan 1;113(1):80-5.
Geiss T, Schaefert RM, Berens S, Hoffmann P, Gauss A. Risk of depression in patients with inflammatory bowel disease. Journal of digestive diseases. 2018 Aug;19(8):456-67.
Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Risk factors for depression in the elderly inflammatory bowel disease population. Journal of Crohn's and Colitis. 2014 Feb 1;8(2):113-9.
Ye Y, Pang Z, Chen W, Ju S, Zhou C. The epidemiology and risk factors of inflammatory bowel disease. International journal of clinical and experimental medicine. 2015;8(12):22529.
M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol 2013; 6: 33-47
Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 2009; 15(7): 1105-18.
Goodwin RD, Dierker LC, Wu M, Galea S, Hoven CW, Weinberger AH. Trends in U.S. Depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63(5):726-733.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56.
Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717-726.
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241-255.
Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96(2):804–10.
Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust. 2009;190(S7):S54–60.
Fang Y, Ding Y, Jinxia D. Advance in stress for depressive disorder. Depress Disord Mech Meas Manag. Singapore: Springer Singapore; 2019. p. 147-78.
Frolkis AD, Vallerand IA, Shaheen A-A, Lowerison MW, Swain MG, Barnabe C, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68(9):1606–12.
Jonefjäll B, Öhman L, Simrén M, Strid H. IBS-like symptoms in patients with ulcerative colitis in deep remission are associated with increased levels of serum cytokines and poor psychological well-being. Inflamm Bowel Dis. 2016;22(11):2630–40.
Colonnello V, Agostini A. Disease course, stress, attachment, and mentalization in patients with inflammatory bowel disease. Med Hypotheses. 2020;140:109665.
Mikocka-Walus A, Ford AC, Drossman DA. Antidepressants in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:184–92.
Bommena S, Goldberg A, Amini M, Alishahi Y. Depression in women with inflammatory bowel disease: a multifaceted approach for a multidimensional problem. Inflammatory Bowel Diseases. 2023 Dec 1;29(12):1957-70.
Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22(3): 752-62.
Angelopoulos NV, MantasC, DalekosGN, et al. Psychiatric factors in patients with ulcerative colitis according to disease activity. Eur J Psychiatry 1996; 10: 87–99.