Research Article - (2024) Volume 19, Issue 6
Role Of Family Physicians In Colorectal Cancer Detection And Prevention A Systematic Review
Anas Salem Alnasiri1*, Faisal Saleh Khalifah Alkhalifah2, Radhi Nasser S Alkubaidan2, Rakan Mohammed Ahmed Alhuwaydi2, Saleh Mohammed saleh Alotaibi2, Ahmed Khalid Ahmed Aljabbab3, Mohammed Abdullah Marzouq Alhadi2, Muath Mohammed S AlZunaydi4 and Mamdouh Madallah A Alsirhani5*Correspondence: Anas Salem Alnasiri, Consultant Family Medicine, Primary Health Care Center, Sakaka, Saudi Arabia, Email:
2General practitioner, King Abdulaziz Specialist Hospital, Sakaka, Saudi Arabia
3Surgery specialist, King Abdulaziz Specialist Hospital, Sakaka, Saudi Arabia
4Registrar General Surgery, King Abdulaziz Specialist Hospital, Sakaka, Saudi Arabia
5Pediatrics Registrar, Urgent Care Center, Sakaka, Saudi Arabia
Received: 05-Dec-2024 Published: 12-Dec-2024
Abstract
Objectives: To investigate literature on the role of family physicians (FPs) related to colorectal cancer (CRC) detection and prevention.
Methods: A total of 513 pertinent publications were found after a comprehensive search across four databases. 46 full-text publications were examined after duplicates were eliminated using Rayyan QCRI and relevance was checked; six studies finally satisfied the requirements for inclusion.
Results: We included six studies with a total of 15,601 participants and more than half of them 9104 (58.4%) were females. The results indicate that PCPs play a pivotal role in CRC screening through counseling, patient education, and the use of diverse screening tools. Effective communication and systematic patient education were found to enhance screening adherence. However, barriers such as insufficient training, lack of resources, and low screening rates in some regions were noted. Availability of colonoscopies and fecal occult blood tests significantly improved screening practices. Regular patient-physician interactions were associated with higher screening rates, highlighting the importance of continuity of care. Addressing gaps in training and resources could further optimize CRCS outcomes.
Conclusion: FPs are pivotal in enhancing CRC screening and prevention through patient education, counseling, and offering diverse screening options. Addressing barriers like inadequate training, resource limitations, and inconsistent practices is essential for improving outcomes. Future research should prioritize longitudinal studies to assess the impact of FPs interventions and develop tailored strategies to overcome systemic challenges. Strengthening primary care systems to support FPs can significantly reduce CRC incidence and mortality.
Keywords
Colorectal cáncer, Screening, Detection, Prevention, Primary care, Family physicians, Family medicine, Systematic review
Introduction
For both men and women, CRC is the third most common cause of cancer-related deaths in the US [1]. With an anticipated 153,000 new cases in 2023 alone, CRC will affect 4.1% of individuals at some point in their lives [2]. The annual death rate is 13 [1] per 100,000 adults, with 36.6 new cases per 100,000. It is anticipated that CRC will account for 8.6% of cancer-related fatalities and 7.8% of all new cancer diagnoses in 2023. Despite these figures, just 72 percent of US adults have had their CRC screenings completed, and for Asian, American Indian, and Alaskan Native people, screening rates have fallen to less than 62 percent [3].
The 5-year survival rate for patients with localized CRC is 90% if the illness is identified early [4]. The United States Preventative Service Task Force (USPSTF) revised its recommendations in 2021 to suggest that healthy adults with average risk start screening for CRC at age 45 instead of 50, as was previously advised, in order to increase early disease detection [5].
In recent years, it has become clear how crucial primary care physicians (PCPs) are to the prevention, diagnosis, and treatment of both benign and malignant gastrointestinal illnesses. With appropriate screening programs, the incidence of CRC might be significantly reduced, making PCPs' involvement even more crucial. Nonetheless, the function of PCPs varies depending on the screening program in each nation or area. With the implementation of population-based programs in several European nations, PCPs now play a more supportive, educational, or facilitating role rather than doing screening [6].
CRC represents one of the most important causes of morbidity and mortality for all cancers worldwide and, thus, has enormous public health implications. The survival rates and healthcare expenditure due to the disease have been proved to show remarkable improvement if the case detection and prevention start early. FPs can play a very important role in early identification of the risk group, advising on proper screening modalities, and conducting prevention. However, the scope and effectiveness of their contributions to CRC detection and prevention have not been comprehensively reviewed. A systematic review of their role can illuminate gaps in practice, identify best practices, and highlight opportunities for improving CRC outcomes through enhanced primary care interventions. It therefore sets out to conduct a systematic review and synthesis of literature on the role of FPs related to CRC detection and prevention in contributing to early screening, risk assessment, patient education, and implementation of preventive strategies.
Methods
Search strategy
The PRISMA and GATHER criteria were adhered to in the systematic review. To locate pertinent research on the role of FPs related to CRC detection and prevention, a comprehensive search was carried out. Four electronic databases were searched by the reviewers: SCOPUS, Web of Science, Cochrane, and PubMed. We eliminated any duplicates and uploaded all of the abstracts and titles that we could find using electronic searches into Rayyan. After that, all of the study texts that met the requirements for inclusion based on the abstract or title were gathered for a thorough examination. Two reviewers independently assessed the extracted papers' suitability and discussed any discrepancies.
Study population-selection
The PEO (Population, Exposure, and Outcome) factors were implemented as inclusion criteria for our review: (i) Population: PCPs or patients eligible for CRC screening, particularly those managed in primary care settings FPs, (ii) Exposure: Interventions, practices, or roles undertaken by FPs, (iii) Outcome: Enhanced early detection of CRC, increased adherence to screening guidelines, improved patient awareness and education regarding prevention, and overall reduction in CRC incidence and mortality.
Data extraction
Data from studies that satisfied the inclusion requirements were extracted by two objective reviewers using a predetermined and uniform methodology. The following information was retrieved and recorded: (i) First author (ii) Year of publication, (iii) Study design, (iv) Country, (v) Sample size, (vi) Age, (vii) Gender, (viii) Main outcomes.
Quality review
Since bias resulting from omitted factors is frequent in studies in this field, we used the ROBINS-I technique to assess the likelihood of bias since it enables a thorough examination of confounding. The ROBINS-I tool can be used for cohort designs where individuals exposed to different staffing levels are tracked over time and is designed to assess non-randomized studies. Each paper's risk of bias was evaluated independently by two reviewers, and any differences were settled by group discussion [7].
Results
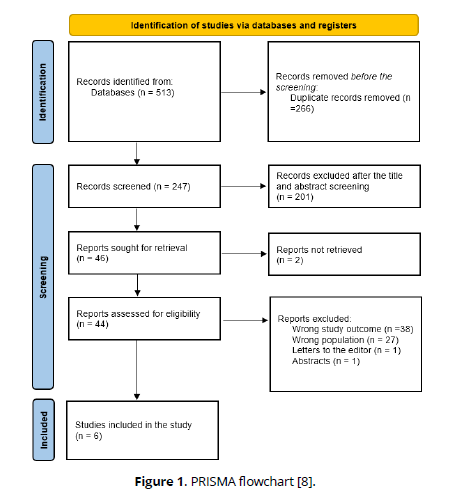
The specified search strategy yielded 513 publications (Figure 1). After removing duplicates (n =266), 247 trials were evaluated based on title and abstract. Of these, 201 failed to satisfy eligibility criteria, leaving just 46 full-text articles for comprehensive review. A total of 6 satisfied the requirements for eligibility with evidence synthesis for analysis.
Figure (1): PRISMA flowchart [8].
Sociodemographic and clinical outcomes
We included six studies with a total of 15,601 participants and more than half of them 9104 (58.4%) were females. Regarding study designs, all of the included studies were cross-sectionals [9-14].
Physicians who can counsel and are supported with the availability of tools such as brief promotion scripts are more likely to increase patient participation in screening programs. It is clear that patient education plays a major role, since systematic communication means that individuals are informed about the procedures for screening, thus enhancing adherence and optimizing outcomes [9].
It concludes with a recurring theme being required educations for PCPs to maintain higher bases and practice of effective screening measures. Despite programs being readily available in some regions, these screening rates are low and suffer from many barriers against availability like a lack of training, resourced areas and facility capability [10]. Additionally, offering choices where clinical facility access to colonoscopies, along with the availability for blood tests, can greatly attribute to success [11].
The studies also focus on the physician's perspective and decision-making factors that influence screening rates. For example, physicians who perceive the benefits of screening modalities from a cost-effective perspective as well as mortality reduction are most likely to recommend them. It was also determined that patient preference and practical considerations, such as waiting times for procedures, did not influence physicians' decisions to recommend screening [12, 13].
Another striking finding is the association between regular patient-physician contact and higher screening rates. Individuals with continuous contact with their FPs are more likely to report engaging in CRC screening, reinforcing the concept of continuity of care as integral to prevention. These deficiencies in training and resources, combined with stimulating regular communication, may substantially improve CRC prevention and early detection [14] (Table 1, Table 2).
| Study ID | Study design | Country | Sociodemographic | Main outcomes |
|---|---|---|---|---|
| Scheid et al., 2013 [9] | Cross-sectional | USA | N= 1118 Mean age: 64 Females: 738 (62.3%) |
Skilled PCPs employ counseling strategies and short CRCS promotion scripts to enhance CRCS performance. |
| Poroes et al., 2020 [10] | Cross-sectional | Switzerland | N= 178 Females: 75 (42.1%) |
Most FPs reported CRC screening procedures that aligned with the program's goals. All patients must be methodically informed about the program, though, in order to guarantee that they are well-informed and to save time. Additionally, FPs must to be urged to provide a variety of tests. |
| Deobald et al., 2013 [11] | Cross-sectional | Canada | N= 339 Females: 131 (38.6) |
FPs in Saskatchewan are now screening for CRC at a low rate. There are educational opportunities about proper screening techniques and advice about populations that are at risk. |
| Zettler et al., 2010 [12] | Cross-sectional | Canada | N= 450 Mean age: Females: 243 (54%) |
The preferences of PCPs and their assessments of patient preferences for CRC screening differed significantly (P <.001). Physicians' perspectives regarding the cost-effectiveness and mortality reduction of the screening modality, as well as their assessments of FOBT sensitivity, affected the screening decision. Wait times for colonoscopies had no bearing on the screening decisions made by doctors. |
| Zarychanski et al., 2007 [13] | Cross-sectional | Canada | N= 12,776 | Self-reported screening rates for CRC are much below acceptable thresholds. A history of current CRC screening is more likely to be reported by individuals who have greater contact with their FP than by those who do not. |
| Females: 7243 (56.7%) | ||||
| Dzhemiliev et al., 2024 [14] | Cross-sectional | Ukraine | N= 740 Females: 674 (91%) |
With 75% of PCPs sending patients for this screening method, respondents (80%) thought colonoscopies were successful in lowering CRC mortality. Lack of resources and PCPs' insufficient screening training were noted as major obstacles. When colonoscopies and fecal occult blood tests were reportedly available in their practices, respondents reported using them extensively for screening. |
| Study ID | Bias due to confounding | Bias in the selection of participants into | Bias in the classification of interventions | Bias due to deviations from the intended interval | Bias due to missing data | Bias in the measurement of outcomes | Bias in the selection of reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Scheid et al., 2013 [9] | Mod | Mod | Low | Low | Low | Low | Low | Low |
| Poroes et al., 2020 [10] | Low | Low | Low | Low | Low | Mod | Low | Low |
| Deobald et al., 2013 [11] | Mod | Low | Mod | Mod | Low | Low | Low | Moderate |
| Zettler et al., 2010 [12] | Low | Low | Mod | Mod | Low | Low | Mod | Moderate |
| Zarychanski et al., 2007 [13] | Mod | Mod | Low | Low | Low | Mod | Mod | Moderate |
| Dzhemiliev et al., 2024 [14] | Mod | Low | Mod | Mod | Low | Low | Low | Moderate |
Discussion
This systematic review emphasizes the fact that FPs play an important role in CRC screening and prevention. The ability of the FPs to counsel, educate, and offer choices of various testing options significantly enhances the screening rates and early detection. However, several challenges are still there which limit the effectiveness, including insufficient training and resource constraints, and variation in adherence to screening programs. Regular contact between patients and FPs was also identified as an important factor in increasing screening uptake, again reflecting the importance of continuity of care. The findings indicate that interventions are needed to enhance physician training, increase resources, and support comprehensive patient education strategies.
Ranies et al. reported that FPs are essential in helping their patients learn how to reduce these risks. FPs must also inform their patients on the various screening methods, their advantages and disadvantages, and the proper clinical indications for each test depending on the risk factors unique to each patient [15].
When it comes to health maintenance checkups, FPs are essential. Patient compliance with CRC screening is influenced by a number of factors, such as the dangers associated with anesthesia, the need for bowel preparation, and uncertainty about the various screening methods. The recommended age for screening for CRC has just been lowered from 50 to 45, however many people are not aware of this change. A lot of people dislike some parts of colonoscopy screening and don't know of other possibilities. If a patient is asymptomatic and has no family history, they often do not perceive the necessity for screening. In other cases, patients skip screening because they are afraid of the money and effort (cost of bowel prep, time away from work, hiring a vehicle). Some people just put off screening because they are lazy [16].
For colorectal screening, the USPSTF suggests grade A for individuals aged 50 to 75, grade B for those aged 45 to 49, and grade C for those aged 76 to 85. There are various screening methods available. The dangers, advantages, and customized screening choices for each patient depending on their personal and family history must be explained by FPs [17].
Rather than following scientific recommendations, the role of PCPs in each nation is determined by the regulations of an established national or regional program. Numerous studies demonstrate that the usage of CRC screening methods varies greatly by geography both within and between nations, and this diversity is primarily explained by local medical cultures, physician preferences, and resource availability. There may be more differences in CRC screening practices and recommendations within each continent than between the USA and Europe, although systematic screening appears to be given more importance in Europe, which helps with quality control. If operating screening programs are viewed as natural platforms for trying out and evaluating expected improvements in the service, including new coming screening modalities, the much-discussed necessity for randomized trials as new screening modalities develop could be handled more readily [18].
A physician called a PCP is in charge of preventing practically all illnesses in people. As a result, this function is the most crucial component of all sophisticated primary care healthcare systems. Patients' survival may be significantly impacted if a PCP fails to advise them of the availability and value of routine screening tests, which could cause major delays in early cancer diagnosis [18].
These findings suggest that PCPs are in need of targeted training programs with appropriate resources, which may enhance CRC prevention. The integration of structured counseling tools and patient education materials within the practice of primary care can improve adherence to guidelines on screening. Policymakers and healthcare systems also need to address systemic barriers, including resource limitations and procedural delays, as ways of optimizing CRCS outcomes. In addition, increased continuity of care may enhance patient-physician relationships, thus leading to more active preventive screening.
Strengths and limitations
This review provides a comprehensive synthesis of current evidence on the role of FPs in CRC detection and prevention. By including studies across multiple countries, it offers insights into global practices and highlights common challenges and opportunities. The use of diverse outcomes ensures a holistic understanding of how FPs contribute to CRCS efforts.
Most of the studies in the review were cross-sectional; hence, establishing causality between FPs interventions and improvement in CRCS outcomes was difficult. Variations in the healthcare system and guidelines on screening in different countries can affect generalizability. The presence of reporting bias and methodological inconsistencies in measuring physicians' practices and patients' outcomes further constrains the strength of conclusions.
Conclusion
FPs play the central role in the improvement of CRC screening and prevention by educating, counseling, and providing options for screening to patients. The removal of barriers, such as lack of training, resources, and inconsistent screening practices, would greatly enhance the outcomes. Longitudinal studies should be the focus of future research in order to determine the causal impact of interventions provided by FPs in CRC prevention and explore tailored strategies for overcoming systemic challenges. Strengthening the primary care systems to support FPs will reduce the incidence and mortality rates of CRC.
References
Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458-79.
SEER Cancer Stat Facts: Colorectal Cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed December 14, 2023.
Centers for Disease Control and Prevention. Use of Colorectal Cancer Screening Tests.,https://www.cdc.gov/cancer/colorectal/statistics/usescreening-tests-BRFSS.htm. Accessed December 14, 2023.
American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Atlanta: American Cancer Society; 2020.
US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977.
Triantafillidis JK, Vagianos C, Gikas A, Korontzi M, Papalois A. Screening for colorectal cancer: the role of the primary care physician. European Journal of Gastroenterology & Hepatology. 2017 Jan 1;29(1):e1-7.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. bmj. 2016 Oct 12;355.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015 Dec 4:1-9.
Scheid DC, Hamm RM, Ramakrishnan K, McCarthy LH, Mold JW. Improving colorectal cancer screening in family medicine: an Oklahoma physicians resource/research network (OKPRN) study. The Journal of the American Board of Family Medicine. 2013 Sep 1;26(5):498-507.
Poroes C, Cornuz J, Gouveia A, Ducros C, Selby K. Self-reported screening practices of family physicians participating in the colorectal cancer screening program of the canton of Vaud: a cross-sectional study. BMC family practice. 2020 Dec 21:1-8.
Deobald R, Graham P, Chad J, Di Gregorio C, Johnstone J, Balbuena L, Kenyon C, Lees M. Colorectal cancer screening practices in Saskatchewan: Survey of family physicians. Canadian Family Physician. 2013 Dec 1;59(12):e558-63.
Zettler M, Mollon B, da Silva V, Howe B, Speechley M, Vinden C. Family physicians’ choices of and opinions on colorectal cancer screening modalities. Canadian Family Physician. 2010 Sep 1;56(9):e338-44.
Zarychanski R, Chen Y, Bernstein CN, Hébert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behaviour. Cmaj. 2007 Sep 11;177(6):593-7.
Dzhemiliev A, Kizub D, Wanis KN, Allar BG, Vus V, Malovanna A, Huivaniuk I, Kopetskyi V, Beznosenko A, Shabat G, Antoniv M. Factors Affecting Colorectal Cancer Screening in Primary Care Physician Practices in Ukraine. JCO Global Oncology. 2024 Aug;10:e2400053.
Raines S, Dennison M, Brondhaver T, Hayes B. Colorectal Cancer Guide for Family Physicians. Osteopathic Family Physician. 2024 May 29;16(2):25-8.
Cooper CP, Gelb CA. Opportunities to expand colorectal cancer screening participation. J Womens Health (Larchmt). 2016;25(10):990-5.
USPSTF.Colorectal cancer: screening. https://www. uspreventiveservicestaskforce.org/uspstf/recommendation/colorectalcancer-screening. Accessed December 1, 2024.
Hoff G, Dominitz JA. Contrasting US and European approaches to colorectal cancer screening: ¿which is best? Gut 2010; 59:407–414.