Research Article - (2025) Volume 20, Issue 1
THE ROLE OF PHARMACOLOGICAL INTERVENTIONS IN OPTIMIZING GLYCEMIC CONTROL AND REDUCING COMPLICATIONS IN DIABETES MELLITUS: CURRENT INSIGHTS AND FUTURE DIRECTIONS
Abdulkareem A. Alanezi**Correspondence: Abdulkareem A. Alanezi, Department of Pharmaceutics, College of Pharmacy, University of Hafr Al Batin, Hafr Al Batin, 39524, Saudi Arabia, Email:
Received: 27-Jan-2025 Published: 12-Feb-2025
Abstract
Diabetes mellitus is a chronic metabolic disorder that affects millions of people worldwide, with its associated complications presenting significant challenges for both individuals and healthcare systems. Effective pharmacological management is essential for achieving optimal glycaemic control, which is key to preventing microvascular issues like retinopathy, nephropathy, and neuropathy, as well as macrovascular problems such as cardiovascular disease and stroke. Recent advancements in diabetes treatment have been driven by the introduction of novel drug classes, including sodium-glucose co-transporter 2 (SGLT2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists, which not only improve glycaemic control but also offer protective effects for the heart and kidneys. While traditional medications, like metformin, sulfonylureas, and insulin, continue to play a central role, their effectiveness is limited by potential side effects, such as hypoglycaemia and weight gain. Ground-breaking clinical trials, including DCCT, UKPDS, EMPA-REG OUTCOME, LEADER, and SONAR have demonstrated the critical link between pharmacological treatments and the reduction of diabetes-related complications. This review examines the current understanding of the mechanisms, effectiveness, and safety of antidiabetic medications, with a focus on their long-term benefits. It also explores emerging trends, such as personalized medicine, digital health innovations, and new therapeutic targets that aim to address remaining cardiovascular and microvascular risks. As the global burden of diabetes continues to rise, there is a pressing need for tailored pharmacological strategies, guided by evidence-based guidelines and individualized patient needs, to improve outcomes and quality of life. This review highlights the importance of ongoing innovation and collaborative efforts to tackle the complex challenges posed by diabetes and its complications.
Keywords
Diabetes Mellitus; Glycaemic Control; Pharmacological Interventions; Antidiabetic Drugs
Introduction
Diabetes mellitus (DM) refers to a group of chronic metabolic disorders characterized by elevated blood glucose levels, resulting from impairments in insulin production, insulin action, or both [1]. The two main forms of diabetes are Type 1 Diabetes mellitus (T1DM) and Type 2 Diabetes mellitus (T2DM). T1DM is an autoimmune disorder where the immune system attacks the insulin-producing beta cells in the pancreas, leading to little or no insulin production [2]. T1DM typically develops during childhood or adolescence but can occur at any age, requiring lifelong insulin therapy [3]. In contrast, T2DM is the most prevalent form, accounting for over 90-95% of diabetes cases [4]. It arises from insulin resistance, where the body's cells do not respond effectively to insulin, often combined with relative insulin deficiency [5]. T2DM is frequently associated with obesity and sedentary behavior, typically manifesting in adults but increasingly diagnosed in younger populations [6]. Management includes lifestyle modifications and, when necessary, oral medications or insulin. Gestational diabetes mellitus (GDM) is another type, occurring during pregnancy when blood glucose levels rise above normal but remain below diagnostic thresholds for diabetes [7]. GDM increases the risk of complications during pregnancy and childbirth, predisposing both the mother and child to an elevated risk of developing T2DM later in life [8].
Diabetes is a significant global public health challenge, with its incidence increasing markedly in recent decades. In 2022, approximately 537 million adults aged 18 and older were living with diabetes worldwide, nearly double previous estimates [9]. Notably, over half of adults aged over 30 with the condition are not receiving treatment, highlighting substantial gaps in healthcare access and management [10]. The worldwide prevalence of diabetes escalated from approximately 7% in 1990 to 10.5% in 2021, with the most pronounced increases noted in low- and middle-income countries [11]. Regions including the Pacific Islands, the Caribbean, the Middle East, North Africa, Pakistan, and Malaysia have some of the highest diabetes prevalence rates. Additionally, India, China, and the United States have the largest populations of individuals living with diabetes [12]. Figure 1: Global Distribution of Diabetes Prevalence, Highlighting Regional Disparities (2021).
Figure 1. Projected Increase in Diabetes Prevalence by Region. Reported data and the International Diabetes Federation indicate that diabetes prevalence is expected to increase by 68% in Southeast Asia, 13% in Europe, 134% in Africa, 50% in South and Central America, 24% in North America, 87% in the Middle East and North Africa, and 27% in the Western Pacific. (This figure was created using Microsoft® Word for Mac, Version 16.89.1.)
The rising prevalence of diabetes is strongly associated with factors such as increasing obesity, sedentary lifestyles, and dietary changes [13]. While effective treatments are available, many individuals, particularly in low-income countries, face barriers to accessing essential medications and care [14]. These barriers include supply chain disruptions, high drug costs, and insufficient healthcare infrastructure. The economic impact of diabetes is substantial, encompassing both direct medical costs and indirect expenses due to reduced productivity [15]. To mitigate this growing challenge, a holistic approach is needed, emphasizing prevention, early detection, and equitable access to quality care and treatment worldwide.
Maintaining proper glycemic control is crucial in diabetes management to prevent or delay complications [16]. Research demonstrates that optimal blood glucose management reduces the risk of microvascular complications, such as retinopathy, neuropathy, and nephropathy, in individuals with both type 1 and type 2 diabetes [17]. Furthermore, improved glycemic control has been associated with a reduction in macrovascular complications, including cardiovascular events, particularly in patients recently diagnosed with diabetes [18]. Inadequate glycemic control accelerates the development of diabetesrelated comorbidities. Therefore, achieving and sustaining target blood glucose levels through a combination of lifestyle modifications and pharmacotherapy is vital for improving quality of life and reducing the healthcare costs associated with diabetes complications.
Pharmacological treatments play a vital role in managing diabetes mellitus, aiming to achieve effective glycemic control and prevent complications. In type 1 diabetes, insulin therapy remains the cornerstone of treatment, replacing the body's inability to produce insulin [19]. For type 2 diabetes, a range of oral and injectable medications are used to enhance insulin sensitivity, stimulate insulin secretion, or reduce hepatic glucose production [20]. Metformin is typically the first-line treatment due to its proven efficacy and safety [21]. Other medications, such as sulfonylureas, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors, are selected based on each patient's specific needs and comorbid conditions [22]. Certain GLP-1 receptor agonists and SGLT2 inhibitors also offer cardiovascular benefits in addition to their role in glycemic control [23]. Selecting the appropriate pharmacological treatment should be personalized, considering factors such as efficacy, potential adverse effects, patient preferences, and cost, to optimize blood glucose management and minimize the risk of diabetes-related complications.
This review aims to provide a comprehensive examination of diabetes mellitus, focusing on its different forms, its global impact, the importance of glycemic control in preventing complications, and the role of pharmacological treatments in managing the condition. The review will cover the pathophysiology of various types of diabetes, global prevalence trends, strategies for maintaining optimal blood glucose levels, and a survey of current pharmacological therapies. By integrating these elements, the review seeks to highlight the multifaceted challenges of managing diabetes and offer recommendations for improving patient outcomes.
Pathophysiology of Diabetes Mellitus
In T1DM, an autoimmune reaction results in the destruction of pancreatic β-cells, leading to absolute insulin deficiency. Genetic predisposition, along with environmental factors, initiates the immune-mediated destruction of β-cells, resulting in the body's inability to produce insulin [24]. Conversely, T2DM is characterized by a combination of insulin resistance and impaired insulin secretion. Peripheral tissues, including muscle and adipose tissue, demonstrate reduced sensitivity to insulin, necessitating increased insulin production. Over time, pancreatic β-cells become dysfunctional, leading to relative insulin deficiency [25]. Contributing factors include genetic susceptibility, obesity, physical inactivity, and poor dietary habits. Figure 2 illustrates the distinct pathophysiological mechanisms that differentiate Type 1 diabetes mellitus (T1DM) from Type 2 diabetes mellitus (T2DM) [26].
Figure 2. Pathogenesis of Type I and Type II Diabetes Mellitus. The left panel illustrates the pathogenesis of Type I diabetes mellitus, where macrophages, T-cells, and inflammation attack the pancreas, leading to the destruction of beta cells and resulting in absolute insulin deficiency. In contrast, the right panel depicts the pathogenesis of Type II diabetes mellitus. Hyperglycemia, hypertriglyceridemia, and inflammatory cytokines impair pancreatic function, reducing beta cell activity and leading to decreased insulin secretion. This insufficient insulin release prevents proper glucose uptake by tissues, contributing to insulin resistance, a key risk factor in the development of Type II diabetes mellitus. (This figure was created using Microsoft® Word for Mac, Version 16.89.1.)
Role of hyperglycaemia in long-term complications
Chronic hyperglycemia in diabetes is a pivotal factor in the development of long-term complications [27]. Elevated glucose levels induce oxidative stress and activate several detrimental processes, including the polyol pathway, the formation of advanced glycation end products (AGEs), the activation of protein kinase C (PKC) isoforms, and the hexosamine pathway [28]. These mechanisms lead to endothelial dysfunction, inflammation, and tissue damage, resulting in microvascular complications (retinopathy, nephropathy, neuropathy) and macrovascular complications (cardiovascular disorders) [29].
Therapeutic strategies targeting glycemic control are essential to mitigate these complications. In T1DM, exogenous insulin administration remains the cornerstone of treatment, aiming to mimic physiological insulin secretion [3]. Advancements in insulin analogs and delivery systems have enhanced glycemic management. For T2DM, a multifaceted approach is employed, including lifestyle modifications and pharmacotherapy [30]. Pharmaceuticals, including metformin, sulfonylureas, thiazolidinediones, DPP-4 inhibitors, GLP- 1 receptor agonists, and SGLT2 inhibitors, address multiple facets of glucose metabolism, encompassing insulin sensitivity, insulin secretion, and renal glucose reabsorption [31]. Individualized treatment plans are formulated based on patient-specific factors to achieve optimal glycemic control and reduce the risk of complications.
Current Pharmacological Interventions
Insulin Therapy
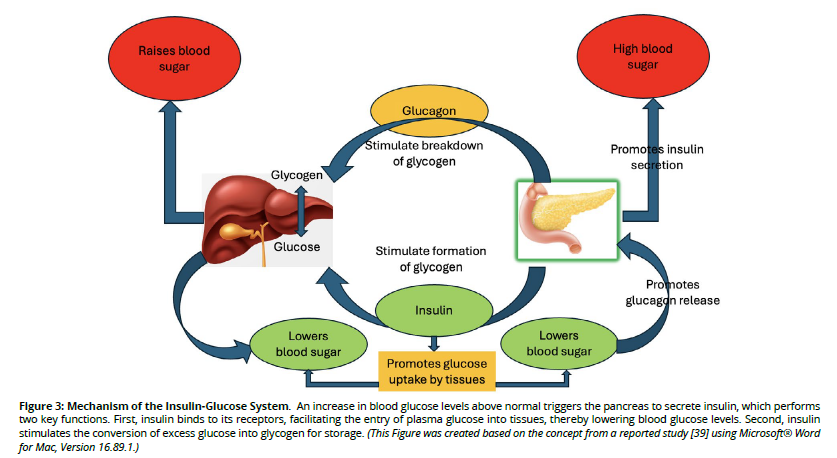
The association of insulin and glucose is illustrated in Figure 3. Insulin therapy remains a cornerstone in the management of DM, with significant advancements enhancing its efficacy and patient adherence [32]. Recent developments in insulin formulations have led to the creation of ultra-longacting analogs, such as insulin degludec, which provide a stable basal insulin level with reduced dosing frequency [33]. Additionally, rapid-acting analogs, including faster aspart, offer improved postprandial glucose control by more closely mimicking physiological insulin secretion [30].
Figure 3. Mechanism of the Insulin-Glucose System. An increase in blood glucose levels above normal triggers the pancreas to secrete insulin, which performs two key functions. First, insulin binds to its receptors, facilitating the entry of plasma glucose into tissues, thereby lowering blood glucose levels. Second, insulin stimulates the conversion of excess glucose into glycogen for storage. (This Figure was created based on the concept from a reported study [39] using Microsoft® Word for Mac, Version 16.89.1.)
Innovations in insulin delivery systems have further improved diabetes management. Insulin pens have simplified administration, enhancing dosing accuracy and convenience [34]. Insulin pumps allow for continuous subcutaneous insulin infusion, providing flexibility in basal and bolus dosing [35]. The advent of closed-loop systems, or artificial pancreas devices, integrates continuous glucose monitoring with automated insulin delivery, maintaining glucose levels within target ranges with minimal patient intervention [36].
Despite these advancements, challenges persist in insulin therapy. Hypoglycemia remains a significant concern, particularly with intensive insulin regimens aimed at tight glycemic control [37]. Weight gain is another common adverse effect, potentially exacerbating insulin resistance and complicating diabetes management [38]. Patient adherence is influenced by factors such as the complexity of insulin regimens, fear of injections, and the psychological burden of chronic disease management [38]. Addressing these challenges requires a comprehensive approach, including patient education, individualized therapy plans, and ongoing support to optimize treatment outcomes. Figure 3 illustrates the process of insulin and glucose interaction [39].
Oral Antidiabetic Drugs (OADs)
Oral antidiabetic drugs (OADs) play a pivotal role in managing T2DM, with various classes targeting distinct pathophysiological aspects of the disease [40].
Sulfonylureas, such as glipizide and glyburide, function by stimulating pancreatic β-cells to release insulin [41]. They accomplish this by binding to sulfonylurea receptors on β-cells, resulting in the closure of ATP-sensitive potassium channels, membrane depolarization, and subsequent insulin release [42]. Sulfonylureas are clinically effective in lowering blood glucose levels; however, their use carries a risk of hypoglycemia and weight gain. Moreover, some studies have indicated a heightened risk of adverse cardiovascular outcomes associated with sulfonylurea medication [43].
Metformin, a biguanide, primarily decreases hepatic gluconeogenesis and enhances insulin sensitivity in peripheral tissues. It activates AMP-activated protein kinase (AMPK), leading to reduced glucose production in the liver. Metformin is widely considered the optimal first-line therapy for patients with T2DM due to its efficacy, low risk of hypoglycemia, and potential cardiovascular benefits [44].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, including empagliflozin and canagliflozin, reduce blood glucose levels by inhibiting renal glucose reabsorption and promoting glycosuria [45]. Beyond glycemic control, SGLT2 inhibitors demonstrate significant cardiovascular and renal benefits. They have shown effectiveness in reducing the risk of hospitalization for heart failure and in slowing the progression of chronic kidney disease in individuals with type 2 diabetes mellitus [46].
Dipeptidyl peptidase-4 (DPP-4) inhibitors, like sitagliptin and saxagliptin, enhance the incretin system by inhibiting the DPP-4 enzyme, which degrades incretin hormones like glucagon-like peptide-1 (GLP-1) [47]. This inhibition leads to increased levels of active incretins, which stimulate glucose-dependent insulin secretion and reduce glucagon release, thereby improving glycemic control [48].
Thiazolidinediones (TZDs), such as pioglitazone, improve insulin sensitivity in peripheral tissues through the activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ) [49]. They can reduce A1c by 0.5–1.4%. Although effective, TZDs are associated with weight gain and have been linked to adverse effects, including fluid retention and an increased risk of heart failure.
Agents like acarbose inhibit intestinal enzymes responsible for carbohydrate digestion, thereby slowing glucose absorption [50]. Common side effects include gastrointestinal disturbances such as flatulence and diarrhea [20].
The choice of suitable OADs must be tailored to individual patient characteristics, encompassing efficacy, adverse effect profiles, comorbidities, and patient preferences. Combining medications with complementary mechanisms of action can improve glycemic control while minimizing unwanted effects. Current research is enhancing the therapeutic approaches for the management of T2DM using OADs.
Limitations of the of the Oral Antidiabetic Agents
OADs are fundamental in managing T2DM, yet they present certain limitations concerning efficacy, disease progression, and β-cell function [51]. Initially, OADs can effectively lower blood glucose levels; however, their efficacy often diminishes over time due to the progressive decline in pancreatic β-cell function inherent in T2DM [52]. This deterioration leads to reduced insulin secretion, necessitating the addition of other medications or insulin therapy to maintain glycemic control [53]. Persistent hyperglycemia, even during OAD therapy, increases the risk of microvascular and macrovascular complications, including retinopathy, nephropathy, neuropathy, and cardiovascular diseases [54]. Moreover, many OADs do not address the continuous decline in β-cell function; for instance, while sulfonylureas stimulate insulin secretion, they may contribute to β-cell exhaustion over time, potentially accelerating β-cell failure [55]. Side effects associated with OADs, such as hypoglycemia with sulfonylureas or gastrointestinal issues with metformin, can also affect patient adherence, leading to suboptimal glycemic control and further exacerbating disease progression and complication risks [56]. Therefore, while OADs are essential in T2DM management, their limitations necessitate ongoing monitoring and potential adjustments in therapeutic strategies to address disease progression, prevent complications, and preserve β-cell function.
Injectable Non-Insulin Therapies
Injectable non-insulin pharmacotherapies have significantly enhanced the management of T2DM, particularly with the introduction of GLP-1 receptor agonists and innovative dual agonists that simultaneously target glucosedependent insulinotropic polypeptide (GIP) and GLP-1 receptors [57].
GLP-1 receptor agonists, such as semaglutide and liraglutide, mimic the incretin hormone GLP-1, enhancing glucose-dependent insulin secretion, suppressing glucagon release, and prolonging gastric emptying [58]. These mechanisms enhance glycemic regulation and facilitate significant weight loss. In addition to metabolic effects, GLP-1 receptor agonists have shown cardiovascular benefits. Clinical trials have demonstrated a reduction in major adverse cardiovascular events (MACE), including myocardial infarction and stroke, among individuals receiving these medications [59]. The cardiovascular protective effects are believed to be mediated by multiple mechanisms, including improvements in endothelial function, reductions in blood pressure, and anti-inflammatory actions [59].
Emerging therapies, such as tirzepatide, act as dual agonists for both GIP and GLP-1 receptors [60]. Tirzepatide has demonstrated greater efficacy in glycemic regulation and weight loss than selective GLP-1 receptor agonists. Dual agonism augments insulin secretion and sensitivity, concurrently fostering satiety, resulting in substantial weight reduction [61]. Clinical studies have indicated that tirzepatide achieves glucose levels akin to diabetes remission and substantial weight reduction, positioning it as a promising therapeutic option for T2DM and obesity management [62].
Pramlintide is an amylin analog used as an adjunct therapy in T2DM [63]. It modulates postprandial glucose levels by slowing gastric emptying, suppressing glucagon secretion, and promoting satiety [64]. While effective in improving postprandial glucose control, pramlintide requires multiple daily injections and careful patient selection due to the risk of hypoglycemia, particularly when used with insulin [64].
In summary, injectable non-insulin therapies, particularly GLP-1 RAs, have expanded the therapeutic options for T2DM management, offering benefits in glycemic control and weight reduction. Ongoing research and development continue to enhance their efficacy and safety profiles, providing promising avenues for comprehensive diabetes care.
Limitations of the of the injectable No-insulin therapies
Injectable non-insulin therapies, such as GLP-1 RAs and amylin analogs, play a significant role in managing T2DM. However, their use is associated with several limitations. Common adverse effects include gastrointestinal disturbances like nausea, vomiting, and diarrhea, which can affect patient adherence to treatment [65]. Additionally, injection site reactions, such as pain, redness, and swelling, may occur, particularly with once-weekly formulations [66]. There are also concerns regarding potential kidney damage associated with GLP-1 RAs, necessitating caution in patients with pre-existing renal impairment [67]. Moreover, the cost of these therapies is generally higher compared to insulin, posing financial challenges for patients and healthcare systems. Furthermore, initiating injectable therapies can be time-consuming for healthcare providers and may require comprehensive patient education to ensure proper administration and adherence. These factors collectively highlight the need for individualized patient assessment when considering injectable non-insulin therapies for diabetes management.
Emerging Therapies
Amylin analogs and other innovative drugs are emerging as prospective therapeutic alternatives for the treatment of numerous conditions, including diabetes and obesity [68]. Amylin, a hormone co-secreted with insulin by the pancreas, is essential in regulating glucose metabolism by delaying gastric emptying, enhancing satiety, and suppressing glucagon release [64]. Amylin analogs, such as pramlintide, mimic these actions and have been used as adjunct therapies to insulin in managing type 1 and type 2 diabetes [69]. Research into other novel agents focuses on targeting new molecular pathways that modulate insulin resistance, glucose homeostasis, and metabolic processes [70]. For instance, drugs that target the GLP-1 receptor, GIP receptor, or inhibit DPP-4 are being explored for their potential to improve glycemic control while offering weight-loss benefits [71]. Table 1 provides a comprehensive list of emerging therapies.
| Therapy | Description | Current Status |
|---|---|---|
| Gene Therapy [72] | Involves modifying or correcting specific genes responsible for insulin production or glucose metabolism to restore normal function | Early-stage research; clinical trials are ongoing to assess safety and efficacy. |
| Stem Cell Therapy [73] | Utilizes stem cells to generate insulin-producing β-cells, aiming to replace damaged or dysfunctional pancreatic cells. | Clinical trials are underway; some patients have shown insulin independence post-treatment. |
| Immunotherapy [74] | Targets the autoimmune response in type 1 diabetes to preserve or restore β-cell function, potentially delaying or preventing disease onset. | FDA-approved therapies like teplizumab have shown promise in delaying type 1 diabetes progression. |
| Artificial Pancreas Systems [75] | Combines continuous glucose monitoring with insulin delivery systems to automate blood sugar regulation, mimicking pancreatic function. | Several systems have received FDA approval and are in use, with ongoing improvements in algorithms and user interfaces. |
| Cell Encapsulation [76] | Encapsulates insulin-producing cells in a protective barrier, allowing insulin release while preventing immune system attacks. | Preclinical and early clinical trials are assessing the long-term viability and functionality of encapsulated cells. |
| Dual- and Triple-Agonist Therapies [77] | Develops drugs that simultaneously activate multiple hormonal pathways (e.g., GLP-1, GIP, glucagon receptors) to improve glucose control and promote weight loss. | Some agents are in late-stage clinical trials, showing promising results in glycemic control and weight reduction. |
| Nanotechnology-Based Delivery Systems [57] | Employs nanoparticles to deliver insulin or other therapeutic agents more effectively, enhancing absorption and reducing side effects. | Experimental stage; research is focused on optimizing delivery mechanisms and ensuring safety. |
Pharmacological Interventions and Complication Prevention
Pharmacological therapies are essential in reducing microvascular and macrovascular complications in individuals with chronic diseases like diabetes [78]. Microvascular complications, such as nephropathy, retinopathy, and neuropathy, are significant contributors to morbidity and mortality, and appropriate pharmacotherapy can substantially mitigate the risk of these complications [79]. Medications such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and SGLT2 inhibitors have proven effective in preventing diabetic nephropathy by reducing blood pressure and mitigating glomerular hyperfiltration [80]. For diabetic retinopathy and neuropathy, interventions that control blood glucose levels and improve vascular health are essential, with clinical studies showing that tight glycemic control can delay or reduce the progression of these complications [81]. Therapies aimed at cardiovascular risk factors, including statins, antihypertensives, and novel medications like GLP-1 receptor agonists and SGLT2 inhibitors, have demonstrated efficacy in reducing the incidence of atherosclerosis, myocardial infarction, and stroke regarding macrovascular outcomes. Significant clinical trials have provided substantial data about the benefits of these medications in improving cardiovascular outcomes for individuals with type 2 diabetes [82]. The EMPA-REG OUTCOME trial indicated that the SGLT2 inhibitor empagliflozin dramatically reduced the risk of major cardiovascular events and the progression of renal disease [83]. Likewise, LEADER and DECLARE-TIMI 58 emphasized the cardiovascular protective benefits of GLP-1 receptor agonists and SGLT2 inhibitors, respectively, underscoring their significance for both glycemic regulation and overall vascular health [84].
These findings highlight the essential significance of pharmacological therapies in mitigating both microvascular and macrovascular complications, thereby enhancing long-term outcomes for patients with diabetes and other metabolic disorders. The relevant clinical trials are listed below in Table 2.
| Trial Name | Population | Drug/Intervention | Outcome | Impact on Complications |
|---|---|---|---|---|
| DCCT (Diabetes Control and Complications Trial) (1993) [85] | Type 1 Diabetes (1,441 participants) | Intensive insulin therapy vs. conventional | Improved glycemic control (A1C ~7% vs. ~9%) | Decreased microvascular consequences (retinopathy, nephropathy, neuropathy). The extended follow-up (EDIC) demonstrated a decrease in macrovascular problems. |
| UKPDS (United Kingdom Prospective Diabetes Study) (1998) [86] | Newly diagnosed Type 2 Diabetes (5,102 participants) | Sulfonylureas, insulin, or metformin | Improved glycemic control with A1C reduction | Reduced microvascular complications. Metformin group showed reduced cardiovascular events in overweight patients. |
| ACCORD (Action to Control Cardiovascular Risk in Diabetes) (2008)[87] | Type 2 Diabetes with elevated cardiovascular risk (10,251 participants) | Intensive glycemic control (A1C <6%) vs. standard control (A1C 7–7.9%) | No significant reduction in cardiovascular events. | Increased all-cause mortality in the intensive group. Raised concerns about over-aggressive glycemic control in high-risk patients. Emphasized individualized therapy. |
| ADVANCE (Action in Diabetes and Vascular Disease) (2008) [88] | Type 2 Diabetes (11,140 participants) | Intensive vs. standard glycemic control | Reduction in microvascular complications | No substantial decrease in macrovascular events or mortality; nevertheless, safer glycemic management techniques were highlighted. |
| VADT (Veterans Affairs Diabetes Trial) (2009) [88] | Type 2 Diabetes with long duration (1,791 participants) | Intensive vs. standard glycemic control | Modest reduction in A1C (1.5% difference) | No significant impact on cardiovascular events or mortality. Benefit in microvascular outcomes observed. |
| EMPA-REG OUTCOME (2015) [83] | Type 2 Diabetes with cardiovascular risk (7,020 participants) | Empagliflozin (SGLT2 inhibitor) vs. placebo | Reduced A1C by ~0.7%. Lowered cardiovascular death by 38%. | Demonstrated significant cardiovascular and renal protective effects, setting the stage for SGLT2 inhibitors as a preferred therapy in high-risk patients. |
| LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) (2016) [89] | Type 2 Diabetes with cardiovascular risk (9,340 participants) | Liraglutide (GLP-1 receptor agonist) vs. placebo | Reduced A1C by ~1%. Lowered cardiovascular death by 22%. | Highlighted GLP-1 receptor agonists’ role in reducing cardiovascular and renal complications while providing glycemic control. |
| CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) (2019) [90] | Type 2 Diabetes with kidney disease (4,401 participants) | Canagliflozin (SGLT2 inhibitor) vs. placebo | Reduced progression of kidney disease and cardiovascular events. | The study found a 30% reduction in the primary composite outcome (end-stage kidney disease, doubling of serum creatinine, renal death, or cardiovascular death) and a 32% lower risk of progression to end-stage kidney disease. Canagliflozin also reduced major cardiovascular events by 20% and hospitalization for heart failure by 39%, while slowing the decline in kidney function. . |
| DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) (2019) [91] | Type 2 Diabetes and heart failure (4,744 participants) | Dapagliflozin (SGLT2 inhibitor) vs. placebo | Reduced heart failure hospitalizations and mortality. | Established SGLT2 inhibitors as a treatment for heart failure with or without diabetes, showcasing pleiotropic benefits. |
| SONAR trial (Study of Diabetic Nephropathy with Atrasentan) (2019) [92] | Patients with type 2 diabetes (T2DM) and chronic kidney disease (CKD) | Atrasentan (0.75 mg/day) | Atrasentan reduced the risk of the primary composite renal endpoint by 35% (HR 0.65; 95% CI, 0.49-0.88; p=0.0047) compared to placebo. However, no significant cardiovascular benefit was observed. | Atrasentan was effective in slowing CKD progression but had a higher risk of fluid retention and heart failure events. This limited its widespread adoption despite its renal benefits. The trial demonstrated the potential role of endothelin receptor antagonists in diabetic nephropathy, but safety concerns remained. |
| The SURPASS-5 Randomized Clinical Trial (2022) [93] | Adults with type 2 diabetes who were inadequately controlled on basal insulin therapy (with or without metformin). | Tirzepatide (5 mg, 10 mg, or 15 mg once weekly) compared to placebo. | HbA1c reduction: Up to 2.1% with tirzepatide 15 mg vs. 0.9% with placebo. | Tirzepatide has shown potential benefits for kidney health, which is critical for preventing or slowing the progression of diabetic nephropathy. |
Pharmacoeconomics and Accessibility
Pharmacoeconomics is essential in evaluating the cost-effectiveness of newer pharmacological agents, especially in managing chronic conditions like diabetes, cardiovascular diseases, and obesity [94]. While these novel therapies, such as SGLT2 inhibitors, GLP-1 receptor agonists, and newer amylin analogs, have demonstrated significant clinical benefits, their high costs often present challenges to widespread adoption. In low- and middle-income countries (LMICs), the affordability and availability of these medications are particularly concerning, as healthcare systems may lack the resources to support their inclusion in national formularies [95]. For instance, a study published in The Lancet Diabetes & Endocrinology estimated price targets for newer antidiabetic drugs to facilitate their inclusion in LMICs' essential medicines lists, highlighting the need for strategic pricing to enhance access [95].
Engaging in negotiations with pharmaceutical companies to secure lower prices and implementing tiered pricing models can make medications more affordable in LMICs [96]. This approach involves setting prices based on the economic status of the country, ensuring that essential medicines are accessible without compromising the sustainability of healthcare systems [97].
Encouraging the production of generic versions of expensive medications can significantly reduce costs [97]. Licensing agreements, such as those between Gilead Sciences and generic manufacturers, enable the production of more affordable versions of drugs like lenacapavir, facilitating access in LMICs [98].
Enhancing health systems through investment in infrastructure, personnel training, and supply chain management can optimize healthcare delivery, decrease total treatment costs, and elevate the quality of care [99]. By implementing these strategies, LMICs can enhance the affordability and availability of newer pharmacological agents, ensuring that patients benefit from the latest advancements in medical science without undue financial burden.
Challenges and Limitations
Managing chronic conditions such as diabetes involves complex treatment regimens that can lead to adherence challenges and potential adverse effects [100]. Potential complications related to antidiabetic medications, their effectiveness during treatment, and clinical trial evidence for these medications are listed in Table 3. Patients often face difficulties with multifaceted medication schedules, which can overwhelm them and result in non-adherence [101]. Additionally, side effects like nausea, fatigue, and hypoglycemia can further discourage consistent medication use [102]. The risk of hypoglycemia and other adverse effects remains a critical concern, especially with certain antidiabetic medications [103]. Episodes of low blood sugar can be dangerous and may lead to severe complications if not promptly addressed. Therefore, careful monitoring and patient education are essential to mitigate these risks. There are also knowledge gaps regarding the long-term effects of novel therapies. Although several pharmaceuticals have demonstrated potential in clinical trials, comprehensive data on their prolonged use is limited. Ongoing research and post-marketing surveillance are necessary to fully understand their long-term safety and efficacy profiles.
| Drug/Class | Complication | Effectiveness | Key Evidence/Trials |
|---|---|---|---|
| Metformin | Cardiovascular events | Reduced myocardial infarction (MI) risk in overweight patients. | UKPDS: Demonstrated reduced macro vascular events in newly diagnosed overweight Type 2 diabetes patients. |
| Cancer | Potential protective effect against certain cancers. | Observational studies suggest reduced cancer incidence in metformin users. | |
| Weight gain | Neutral or slight weight reduction. | Widely documented in clinical practice and trials. | |
| Sulfonylureas | Hypoglycemia | Increased risk of hypoglycemia. | Observed in multiple clinical studies, particularly in elderly patients or those with renal impairment. |
| Cardiovascular events | Neutral to slightly increased risk in some studies. | ACCORD and ADVANCE showed no clear cardiovascular benefit, with safety concerns in high-risk populations. | |
| Insulin | Microvascular complications | Significant reduction in retinopathy and nephropathy. | DCCT: Intensive insulin therapy reduced microvascular complications in Type 1 diabetes. |
| Hypoglycemia | High risk, especially with intensive therapy. | DCCT, UKPDS, and other studies. | |
| SGLT2 Inhibitors | Cardiovascular events | Significant reduction in heart failure and CV death. | EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58 trials demonstrated cardiovascular protection. |
| Renal complications | Reduced progression of diabetic kidney disease. | CREDENCE: Highlighted significant Reno protective effects. | |
| Weight reduction | Modest weight loss was observed. | EMPA-REG OUTCOME and related studies. | |
| GLP-1 Receptor Agonists | Cardiovascular events | Reduced major adverse cardiovascular events (MACE). | LEADER, SUSTAIN-6, and REWIND trials showed reduced CV death and stroke risk. |
| Weight reduction | Significant weight loss. | Consistent finding across GLP-1 RA trials. | |
| Microvascular complications | Evidence of reduced nephropathy progression. | LEADER trial showed a renal benefit. | |
| DPP-4 Inhibitors | Cardiovascular events | Neutral effect on cardiovascular outcomes. | TECOS, SAVOR-TIMI 53, and EXAMINE trials showed no increased risk but no significant CV benefit. |
| Hypoglycemia | Low risk of hypoglycemia. | Observed in multiple trials due to glucose-dependent mechanism of action. | |
| Thiazolidinedione’s (TZDs) | Cardiovascular events | Increased risk of heart failure but reduced stroke. | PROactive: Demonstrated reduced stroke risk but increased heart failure risk. |
Role of the Pharmacist
Pharmacists play a pivotal role in enhancing medication adherence and optimizing patient outcomes [104]. They can provide patient education, counsel on proper medication use, and collaborate with healthcare teams to ensure effective treatment plans. Pharmacist-led interventions have been shown to improve medication refills, glycemic control, and overall adherence [105]. Pharmacists are integral to improving medication adherence and patient education. Subsequent research and policy initiatives must focus on addressing these issues, improving the accessibility and efficacy of treatments, and utilizing novel technologies to elevate patient care.
Future Direction
The future of diabetes pharmacotherapy lies in personalized medicine, where treatments are optimized based on patient-specific factors, including genetic profiles, comorbid conditions, and risk of complications. Additionally, the integration of digital health technologies offers promising avenues for monitoring and managing diabetes, enabling more precise and timely interventions. As research continues to evolve, the focus should remain on developing novel therapeutic options that address the unmet needs of diabetic patients, particularly those at high risk for cardiovascular and renal complications. Precision medicine enables the customization of therapeutics according to individual genetic and phenotypic characteristics, resulting in more personalized and efficacious treatments [106]. Advances in drug delivery systems, such as nanotechnology and oral insulin formulations, aim to enhance patient compliance and therapeutic outcomes [107]. Combination therapies targeting multiple pathways may address comorbid conditions more effectively. Additionally, the integration of artificial intelligence in personalizing pharmacotherapy could revolutionize treatment approaches, making them more precise and patient-specific.
Conclusion
Pharmacological therapies are essential in managing diabetes mellitus, as they play a critical role in optimizing glycemic control to prevent and mitigate both microvascular and macrovascular complications. The development of newer drug classes has significantly enhanced therapeutic outcomes, offering additional benefits beyond glycemic control, including cardiovascular and renal protection. While traditional therapies like metformin, sulfonylureas, and insulin continue to play a fundamental role in diabetes management, their use must be carefully tailored to minimize risks such as hypoglycemia and weight gain. Landmark clinical trials have provided a wealth of evidence supporting the efficacy of these agents in improving long-term patient outcomes, highlighting the importance of individualized treatment strategies. As research continues to evolve, the focus should remain on developing novel therapeutic options that address the unmet needs of diabetic patients, particularly those at high risk for cardiovascular and renal complications. By leveraging existing breakthroughs and promoting innovation, we can enhance the quality of life for individuals with diabetes and mitigate the worldwide impact of this chronic condition.
References
Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomedicine & Pharmacotherapy 2023, 168, 115734.
Syed, F.Z. Type 1 diabetes mellitus. Annals of internal medicine 2022, 175, ITC33-ITC48.
von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037-1048.
Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 2021, 128, 1580-1591.
Tönnies, T.; Brinks, R.; Isom, S.; Dabelea, D.; Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Pihoker, C.; Dolan, L.; Liese, A.D. Projections of type 1 and type 2 diabetes burden in the US population aged< 20 years through 2060: the SEARCH for diabetes in youth study. Diabetes Care 2023, 46, 313-320.
Levy, R.B.; Rauber, F.; Chang, K.; Louzada, M.L.d.C.; Monteiro, C.A.; Millett, C.; Vamos, E.P. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clinical Nutrition 2021, 40, 3608-3614.
Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A clinical update on gestational diabetes mellitus. Endocrine reviews 2022, 43, 763-793.
Modzelewski, R.; Stefanowicz-Rutkowska, M.M.; Matuszewski, W.; Bandurska-Stankiewicz, E.M. Gestational diabetes mellitus—recent literature review. Journal of Clinical Medicine 2022, 11, 5736.
Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. International Journal of Molecular Sciences 2025, 26, 1094.
Indrahadi, D.; Wardana, A.; Pierewan, A.C. The prevalence of diabetes mellitus and relationship with socioeconomic status in the Indonesian population. Jurnal Gizi Klinik Indonesia 2021, 17, 103-112.
Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes research and clinical practice 2022, 183, 109050.
Zhou, T.; Du, S.; Sun, D.; Li, X.; Heianza, Y.; Hu, G.; Sun, L.; Pei, X.; Shang, X.; Qi, L. Prevalence and trends in gestational diabetes mellitus among women in the United States, 2006–2017: a population-based study. Frontiers in endocrinology 2022, 13, 868094.
He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. in vivo 2021, 35, 2503-2511.
Tattersall, R.B.; Matthews, D.R. The history of diabetes mellitus. Textbook of diabetes 2024, 1-21.
Suryasa, I.W.; Rodríguez-Gámez, M.; Koldoris, T. Health and treatment of diabetes mellitus. International journal of health sciences 2021, 5, 1-5.
Dimore, A.L.; Edosa, Z.K.; Mitiku, A.A. Glycemic control and diabetes complications among adult type 2 diabetic patients at public hospitals in Hadiya zone, Southern Ethiopia. PloS one 2023, 18, e0282962.
Yosef, T.; Nureye, D.; Tekalign, E. Poor glycemic control and its contributing factors among type 2 diabetes patients at Adama Hospital Medical College in East Ethiopia. Diabetes, Metabolic Syndrome and Obesity 2021, 3273-3280.
Karunarathna, I. The Impact of Hyperglycemia on Perioperative Outcomes in Patients with Diabetes Mellitus. Uva Clinical Lab. Retrieved from The Impact of Hyperglycemia on Perioperative Outcomes in Patients with Diabetes Mellitus 2024.
Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. The Journal of clinical investigation 2021, 131.
Feingold, K.R. Oral and injectable (non-insulin) pharmacological agents for the treatment of type 2 diabetes. Endotext [Internet] 2024.
Grammatiki, M.; Sagar, R.; Ajjan, R.A. Metformin: is it still the first line in type 2 diabetes management algorithm? Current Pharmaceutical Design 2021, 27, 1061-1067.
Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Molecular metabolism 2021, 46, 101102.
Bertoccini, L.; Baroni, M.G. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Diabetes: from Research to Clinical Practice: Volume 4 2021, 193-212.
Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of type 1 diabetes and gut microbiota role. International Journal of Molecular Sciences 2022, 23, 14650.
Mizukami, H.; Kudoh, K. Diversity of pathophysiology in type 2 diabetes shown by islet pathology. Journal of diabetes investigation 2022, 13, 6-13.
Khin, P.P.; Lee, J.H.; Jun, H.-S. Pancreatic beta-cell dysfunction in type 2 diabetes. European Journal of Inflammation 2023, 21, 1721727X231154152.
Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36-50.
Wei, J.; Tian, J.; Tang, C.; Fang, X.; Miao, R.; Wu, H.; Wang, X.; Tong, X. The influence of different types of diabetes on vascular complications. Journal of diabetes research 2022, 2022, 3448618.
Natorska, J. Diabetes mellitus as a risk factor for aortic stenosis: from new mechanisms to clinical implications. Polish Heart Journal (Kardiologia Polska) 2021, 79, 1060-1067.
Karunarathna, I.; Jayathilaka, P. Comprehensive Management of Type 2 Diabetes Mellitus: From Prevention to Novel Therapeutic Approaches. Uva Clinical Lab. Retrieved from Comprehensive Management of Type 2024, 2.
DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism 2022, 137, 155332.
Sugandh, F.; Chandio, M.; Raveena, F.; Kumar, L.; Karishma, F.; Khuwaja, S.; Memon, U.A.; Bai, K.; Kashif, M.; Varrassi, G. Advances in the management of diabetes mellitus: a focus on personalized medicine. Cureus 2023, 15.
Lee, S.-H.; Yoon, K.-H. A century of progress in diabetes care with insulin: a history of innovations and foundation for the future. Diabetes & metabolism journal 2021, 45, 629-640.
MacLeod, J.; Vigersky, R.A. A review of precision insulin management with smart insulin pens: opening up the digital door to people on insulin injection therapy. Journal of Diabetes Science and Technology 2023, 17, 283-289.
Rimon, M.T.I.; Hasan, M.W.; Hassan, M.F.; Cesmeci, S. Advancements in Insulin Pumps: A Comprehensive Exploration of Insulin Pump Systems, Technologies, and Future Directions. Pharmaceutics 2024, 16, 944.
Dermawan, D.; Purbayanto, M.A.K. An overview of advancements in closed-loop artificial pancreas system. Heliyon 2022, 8.
Mathieu, C. Minimising hypoglycaemia in the real world: the challenge of insulin. Diabetologia 2021, 64, 978-984.
Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discovery Today 2022, 27, 822-830.
Sakulrang, S.; Moore, E.J.; Sungnul, S.; de Gaetano, A. A fractional differential equation model for continuous glucose monitoring data. Advances in Difference Equations 2017, 2017, 1-11.
Birajdar, S.V.; Mazahir, F.; Alam, M.I.; Kumar, A.; Yadav, A.K. Repurposing and clinical attributes of antidiabetic drugs for the treatment of neurodegenerative disorders. European Journal of Pharmacology 2023, 176117.
Tomlinson, B.; Patil, N.G.; Fok, M.; Chan, P.; Lam, C.W.K. The role of sulfonylureas in the treatment of type 2 diabetes. Expert Opinion on Pharmacotherapy 2022, 23, 387-403.
Karle, S.N.; Salve, M.T. COMPUTATIONAL INSIGHTS INTO SULFONYLUREA CONTAINING ANTIDIABETIC DRUGS TARGETING PPAR-γ: A COMPREHENSIVE. pancreas 2023, 4, 5.
Khan, M.F. Diabetes and Antidiabetic Drugs. In Medicinal Chemistry for Pharmacy Students; Bentham Science Publishers: 2024; pp. 220-294.
Di Magno, L.; Di Pastena, F.; Bordone, R.; Coni, S.; Canettieri, G. The mechanism of action of biguanides: New answers to a complex question. Cancers 2022, 14, 3220.
Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: a review. Frontiers in Medicine 2021, 8, 777861.
Forycka, J.; Hajdys, J.; Krzemińska, J.; Wilczopolski, P.; Wronka, M.; Młynarska, E.; Rysz, J.; Franczyk, B. New insights into the use of Empagliflozin—a comprehensive review. Biomedicines 2022, 10, 3294.
Pechmann, L.; Pinheiro, F.; Andrade, V.; Moreira, C. The multiple actions of dipeptidyl peptidase 4 (DPP-4) and its pharmacological inhibition on bone metabolism: a review. Diabetology & Metabolic Syndrome 2024, 16, 175.
Florentin, M.; Kostapanos, M.S.; Papazafiropoulou, A.K. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World Journal of Diabetes 2022, 13, 85.
Mohajan, D.; Mohajan, H.K. Peroxisome Proliferator-Activated Receptor γ (PPAR γ): A Systemic Insulin Sensitizer Associated with Decreased Risk of Type 2 Diabetes. Journal of Innovations in Medical Research 2024, 3, 18-24.
Zamani, M.; Nikbaf-Shandiz, M.; Aali, Y.; Rasaei, N.; Zarei, M.; Shiraseb, F.; Asbaghi, O. The effects of acarbose treatment on cardiovascular risk factors in impaired glucose tolerance and diabetic patients: a systematic review and dose–response meta-analysis of randomized clinical trials. Frontiers in Nutrition 2023, 10, 1084084.
Piragine, E.; Petri, D.; Martelli, A.; Calderone, V.; Lucenteforte, E. Adherence to oral antidiabetic drugs in patients with type 2 diabetes: systematic review and meta-analysis. Journal of Clinical Medicine 2023, 12, 1981.
Lyngbaek, M.P.; Legaard, G.E.; Bennetsen, S.L.; Feineis, C.S.; Rasmussen, V.; Moegelberg, N.; Brinkløv, C.F.; Nielsen, A.B.; Kofoed, K.S.; Lauridsen, C.A. The effects of different doses of exercise on pancreatic β-cell function in patients with newly diagnosed type 2 diabetes: study protocol for and rationale behind the “DOSE-EX” multi-arm parallel-group randomised clinical trial. Trials 2021, 22, 244.
Yadav, J.P.; Maheshwari, S.; Kumar, A.; Jain, N.; Jain, N.; Shukla, S. Advancements in Diabetes Mellitus Treatment: Overcoming Limitations through Innovative Therapeutic Approaches. NeuroQuantology 2022, 20, 7888.
Charles, M.A.; Leslie, R.D. Diabetes: concepts of β-cell organ dysfunction and failure would lead to earlier diagnoses and prevention. Diabetes 2021, 70, 2444-2456.
Miyoshi, H.; Baxter, M.; Kimura, T.; Hattori, M.; Morimoto, Y.; Marinkovich, D.; Tamiwa, M.; Hirose, T. A real-world, observational study of the initiation, use, and effectiveness of basal-bolus or premixed insulin in Japanese people with Type 2 diabetes. Diabetes Therapy 2021, 12, 1341-1357.
Zhang, P.; Chen, M.; Zhang, H.; Luo, Y.; Zhu, D.; Li, X.; Ji, J.; Wang, D.; Duolikun, N.; Ji, L. Effectiveness and safety of basal insulin therapy in type 2 diabetes mellitus patients with or without metformin observed in a national cohort in China. BMC Endocrine Disorders 2022, 22, 26.
Rocha, S.; Lucas, M.; Ribeiro, D.; Corvo, M.L.; Fernandes, E.; Freitas, M. Nano-based drug delivery systems used as vehicles to enhance polyphenols therapeutic effect for diabetes mellitus treatment. Pharmacological Research 2021, 169, 105604.
Yang, Y.; Chen, S.; Liu, Y.; Huang, Y.; Cheong, K.-L.; Teng, B.; Liu, W. Long-term treatment of polysaccharides-based hydrogel microparticles as oral insulin delivery in streptozotocin-induced type 2 diabetic mice. Biomedicine & Pharmacotherapy 2021, 133, 110941.
Rastogi, A.; Sudhayakumar, A.; Schaper, N.C.; Jude, E.B. A paradigm shift for cardiovascular outcome evaluation in diabetes: major adverse cardiovascular events (MACE) to major adverse vascular events (MAVE). Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2023, 17, 102875.
Karagiannis, T.; Avgerinos, I.; Liakos, A.; Del Prato, S.; Matthews, D.R.; Tsapas, A.; Bekiari, E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia 2022, 65, 1251-1261.
Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovascular diabetology 2021, 20, 225.
Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Frontiers in Endocrinology 2024, 15, 1431292.
Panou, T.; Gouveri, E.; Popovic, D.S.; Papanas, N. Amylin analogs for the treatment of obesity without diabetes: present and future. Expert Review of Clinical Pharmacology 2024, 17, 891-899.
Eržen, S.; Tonin, G.; Jurišić Eržen, D.; Klen, J. Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity. International Journal of Molecular Sciences 2024, 25, 1517.
Marathe, C.S.; Rayner, C.K.; Wu, T.; Jones, K.L.; Horowitz, M. Gastrointestinal disorders in diabetes. Endotext [Internet] 2024.
Desai, M.; Kundu, A.; Hageman, M.; Lou, H.; Boisvert, D. Monoclonal antibody and protein therapeutic formulations for subcutaneous delivery: high-concentration, low-volume vs. low-concentration, high-volume. In Proceedings of the MAbs, 2023; p. 2285277.
Liabeuf, S.; Minutolo, R.; Floege, J.; Zoccali, C. The use of SGLT2 inhibitors and GLP-1 receptor agonists in older patients: a debate on approaches in CKD and non-CKD populations. Clinical Kidney Journal 2024, sfae380.
Dehestani, B.; Stratford, N.R.; le Roux, C.W. Amylin as a future obesity treatment. Journal of obesity & metabolic syndrome 2021, 30, 320.
Mathiesen, D.S.; Lund, A.; Vilsbøll, T.; Knop, F.K.; Bagger, J.I. Amylin and calcitonin: potential therapeutic strategies to reduce body weight and liver fat. Frontiers in Endocrinology 2021, 11, 617400.
Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. Recent advances in the treatment of insulin resistance targeting molecular and metabolic pathways: fighting a losing battle? Medicina 2022, 58, 472.
Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Advanced drug delivery reviews 2021, 176, 113888.
Kessler, J.A.; Shaibani, A.; Sang, C.N.; Christiansen, M.; Kudrow, D.; Vinik, A.; Shin, N.; Group, V.S. Gene therapy for diabetic peripheral neuropathy: A randomized, placebo‐controlled phase III study of VM202, a plasmid DNA encoding human hepatocyte growth factor. Clinical and translational science 2021, 14, 1176-1184.
De Klerk, E.; Hebrok, M. Stem cell-based clinical trials for diabetes mellitus. Frontiers in endocrinology 2021, 12, 631463.
Bluestone, J.A.; Buckner, J.H.; Herold, K.C. Immunotherapy: building a bridge to a cure for type 1 diabetes. Science 2021, 373, 510-516.
Moon, S.J.; Jung, I.; Park, C.-Y. Current advances of artificial pancreas systems: a comprehensive review of the clinical evidence. Diabetes & Metabolism Journal 2021, 45, 813-839.
Opara, A.; Jost, A.; Dagogo-Jack, S.; Opara, E.C. Islet cell encapsulation–Application in diabetes treatment. Experimental Biology and Medicine 2021, 246, 2570-2578.
Gutgesell, R.M.; Nogueiras, R.; Tschöp, M.H.; Müller, T.D. Dual and Triple Incretin-Based Co-agonists: Novel Therapeutics for Obesity and Diabetes. Diabetes Therapy 2024, 15, 1069-1084.
Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; moazzam Khan, M.S. Cardiovascular complications of diabetes: from microvascular to macrovascular pathways. Cureus 2023, 15.
Goldney, J.; Sargeant, J.A.; Davies, M.J. Incretins and microvascular complications of diabetes: neuropathy, nephropathy, retinopathy and microangiopathy. Diabetologia 2023, 66, 1832-1845.
Banerjee, D.; Winocour, P.; Chowdhury, T.; De, P.; Wahba, M.; Montero, R.; Fogarty, D.; Frankel, A.; Karalliedde, J.; Mark, P.B. Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC nephrology 2022, 23, 1-31.
Sinclair, S.H.; Schwartz, S. Diabetic retinopathy: New concepts of screening, monitoring, and interventions. Survey of Ophthalmology 2024.
Yu, J.; Zhou, Z.; Mahaffey, K.W.; Matthews, D.R.; Neuen, B.L.; Heerspink, H.J.; Jardine, M.J.; Li, J.; Perkovic, V.; Neal, B. An exploration of the heterogeneity in effects of SGLT2 inhibition on cardiovascular and all-cause mortality in the EMPA-REG OUTCOME, CANVAS Program, DECLARE-TIMI 58, and CREDENCE trials. International Journal of Cardiology 2021, 324, 165-172.
Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New england journal of medicine 2015, 373, 2117-2128.
Mosenzon, O.; Raz, I.; Wiviott, S.D.; Schechter, M.; Goodrich, E.L.; Yanuv, I.; Rozenberg, A.; Murphy, S.A.; Zelniker, T.A.; Langkilde, A.M. Dapagliflozin and prevention of kidney disease among patients with type 2 diabetes: post hoc analyses from the DECLARE-TIMI 58 trial. Diabetes Care 2022, 45, 2350-2359.
Control, D.; Group, C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England journal of medicine 1993, 329, 977-986.
Group, U.P.D.S. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Bmj 1998, 317, 703-713.
Group, A.t.C.C.R.i.D.S. Effects of intensive glucose lowering in type 2 diabetes. New England journal of medicine 2008, 358, 2545-2559.
Group, A.C. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England journal of medicine 2008, 358, 2560-2572.
Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2016, 375, 311-322.
Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England journal of medicine 2019, 380, 2295-2306.
McMurray, J.J.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S. The Dapagliflozin and Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. European journal of heart failure 2019, 21, 1402-1411.
Heerspink, H.J.; Parving, H.-H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.-F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. The Lancet 2019, 393, 1937-1947.
Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. Jama 2022, 327, 534-545.
Xue, Y.; Zou, H.; Ruan, Z.; Chen, X.; Lai, Y.; Yao, D.; Ung, C.O.L.; Hu, H. Pharmacoeconomic evaluation of anti-obesity drugs for chronic weight management: a systematic review of literature. Frontiers in Endocrinology 2023, 14, 1254398.
Ocran Mattila, P.; Ahmad, R.; Hasan, S.S.; Babar, Z.-U.-D. Availability, affordability, access, and pricing of anti-cancer medicines in low-and middle-income countries: a systematic review of literature. Frontiers in Public Health 2021, 9, 628744.
Organization, W.H. Managing conflicts of interest: a how-to guide for public pharmaceutical-sector committees in low-and middle-income countries; World Health Organization: 2022.
Neumann, P.J.; Cohen, J.T.; Ollendorf, D.A. The right price: a value-based prescription for drug costs; Oxford University Press: 2021.
Hill, A.; Levi, J.; Fairhead, C.; Pilkington, V.; Wang, J.; Johnson, M.; Layne, J.; Roberts, D.; Fortunak, J. Lenacapavir to prevent HIV infection: current prices versus estimated costs of production. Journal of Antimicrobial Chemotherapy 2024, 79, 2906-2915.
Organization, W.H. Health systems resilience toolkit: a WHO global public health good to support building and strengthening of sustainable health systems resilience in countries with various contexts. 2022.
Kvarnström, K.; Westerholm, A.; Airaksinen, M.; Liira, H. Factors contributing to medication adherence in patients with a chronic condition: a scoping review of qualitative research. Pharmaceutics 2021, 13, 1100.
Burnier, M. The role of adherence in patients with chronic diseases. European Journal of Internal Medicine 2024, 119, 1-5.
Cucuzzella, M.; Riley, K.; Isaacs, D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Frontiers in nutrition 2021, 8, 688540.
Scheen, A.J. Careful use to minimize adverse events of oral antidiabetic medications in the elderly. Expert Opinion on Pharmacotherapy 2021, 22, 2149-2165.
Paneerselvam, G.S.; Aftab, R.A.; Sirisinghe, R.G.; Lai, P.S.M.; Lim, S.K. Study protocol: Effectiveness of patient centered pharmacist care in improving medication adherence, clinical parameters and quality of life among hemodialysis patients. Plos one 2022, 17, e0263412.
Yousif, O.; Osman, I.; Yousif, M. Pharmacist-Led Intervention on Medication Adherence and Its Impact on Health-Related Quality of Life and Preventing Acute Events Among Diabetic Patients: A Narrative Review. Journal of Acute Care and Resuscitation 2024, 1, 64-69.
Sisodiya, S.M. Precision medicine and therapies of the future. Epilepsia 2021, 62, S90-S105.
Caturano, A.; Nilo, R.; Nilo, D.; Russo, V.; Santonastaso, E.; Galiero, R.; Rinaldi, L.; Monda, M.; Sardu, C.; Marfella, R. Advances in nanomedicine for precision insulin delivery. Pharmaceuticals 2024, 17, 945.